The document provides an overview of Chapter 14 on mixtures and solutions in chemistry. It covers the following key topics:
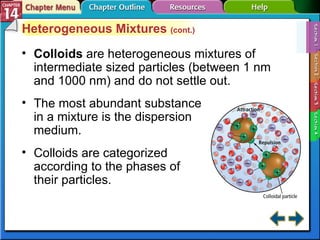
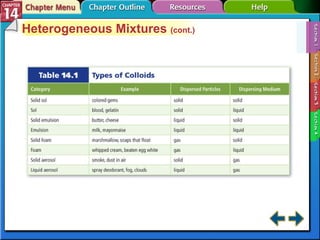
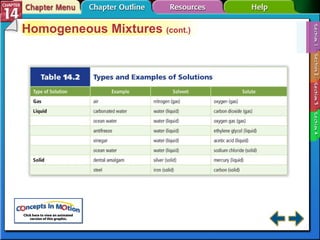
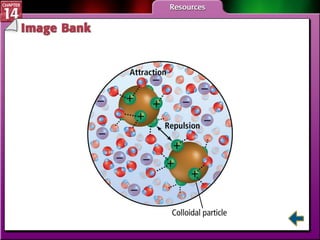
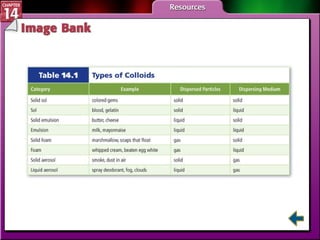
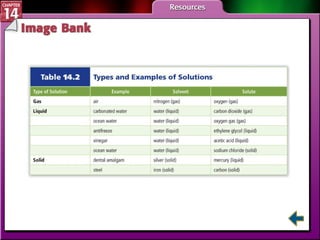
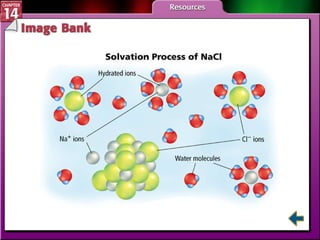
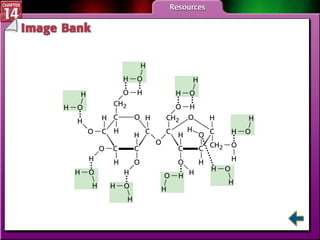
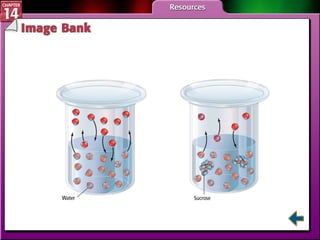
- Section 14.1 defines heterogeneous mixtures like suspensions and colloids, and homogeneous solutions. It describes the properties of different types of mixtures.
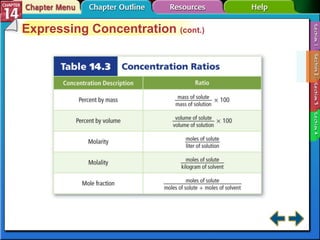
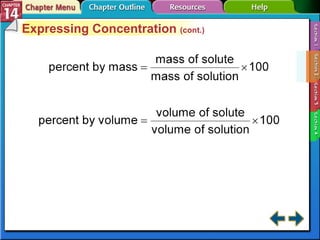
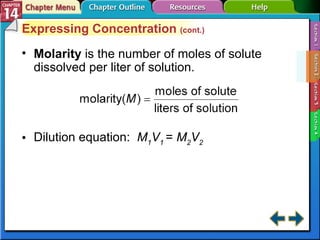
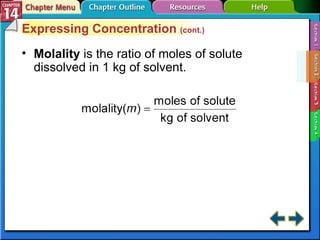
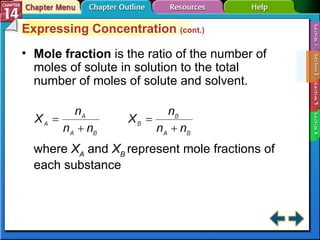
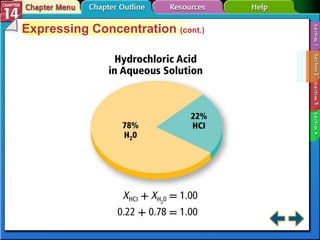
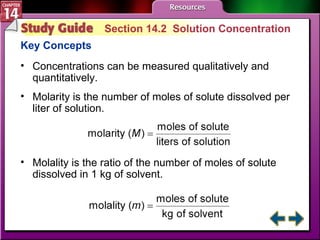
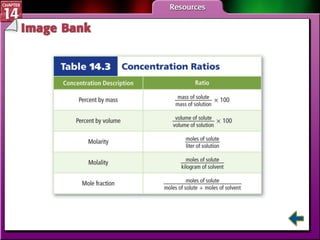
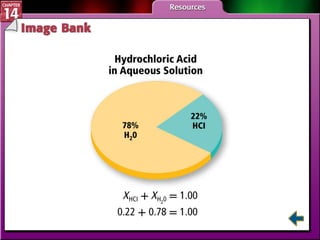
- Section 14.2 discusses various units used to express the concentration of solutions, including molarity, molality, and mole fraction.
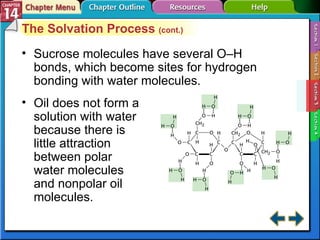
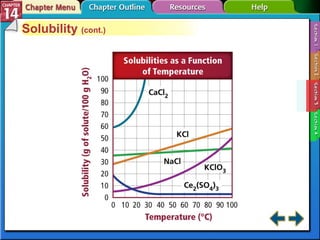
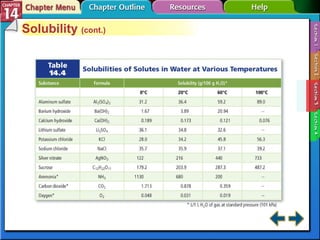
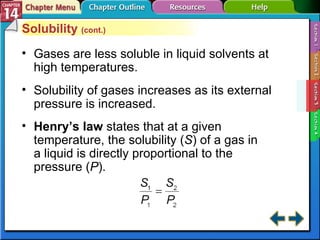
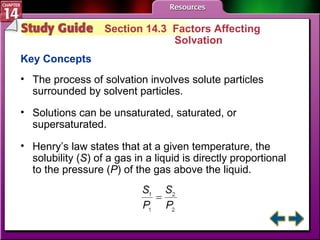
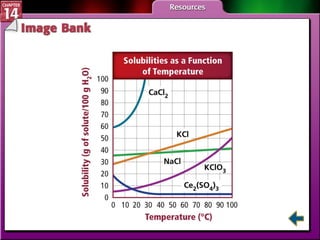
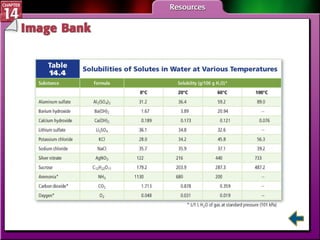
- Section 14.3 examines factors that affect solubility, such as temperature, pressure, and the nature of the solute and solvent. It also defines terms like unsaturated, saturated, and supersaturated solutions.
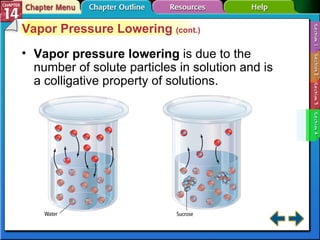
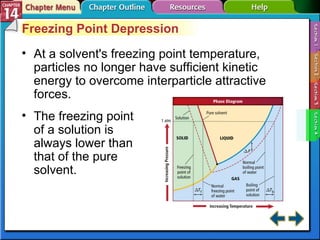
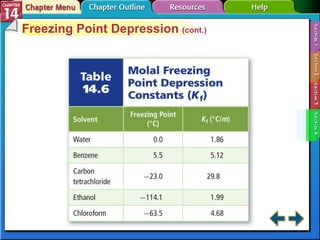
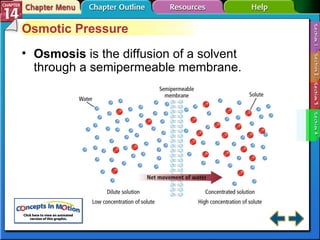
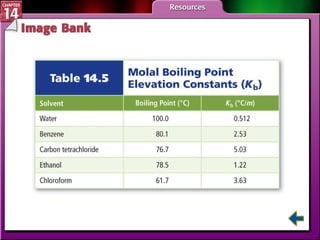
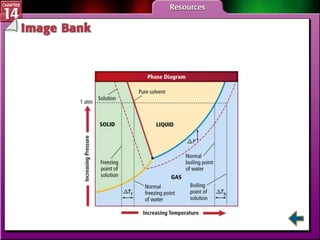
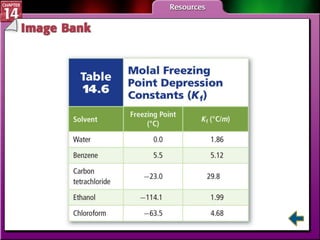
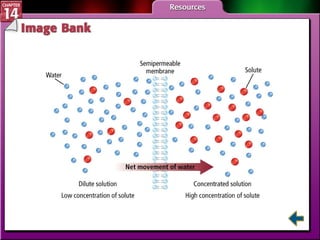
- Section 14.4 introduces colligative properties of solutions that depend on the











































![Help Click any of the background top tabs to display the respective folder. Within the Chapter Outline, clicking a section tab on the right side of the screen will bring you to the first slide in each respective section. Simple navigation buttons will allow you to progress to the next slide or the previous slide. The “Return” button will allow you to return to the slide that you were viewing when you clicked either the Resources or Help tab. The Chapter Resources Menu will allow you to access chapter specific resources from the Chapter Menu or any Chapter Outline slide. From within any feature, click the Resources tab to return to this slide. To exit the presentation, click the Exit button on the Chapter Menu slide or hit Escape [Esc] on your keyboards while viewing any Chapter Outline slide.](https://image.slidesharecdn.com/cmcchapter14-100613133931-phpapp01/85/Cmc-chapter-14-90-320.jpg)
