1) The document provides an overview of key concepts in chemistry including the mole concept, chemical formulas and equations, and different types of chemical reactions.
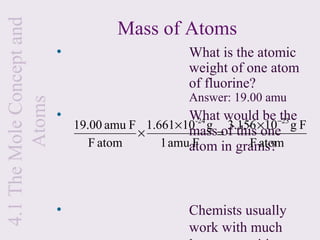
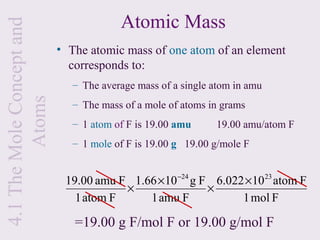
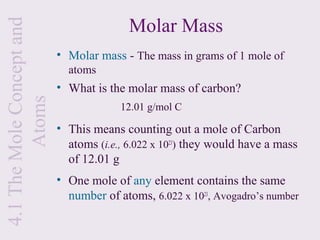
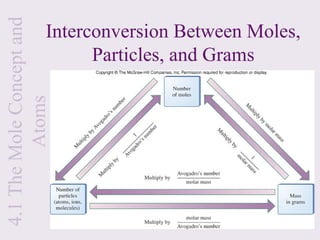
2) It explains that the mole is a unit used to measure amounts of substances and is equal to 6.022x1023 particles. Molar mass refers to the mass of one mole of a substance.
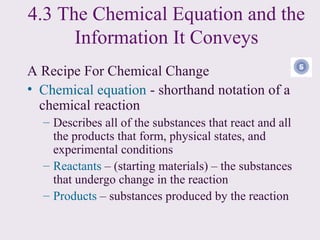
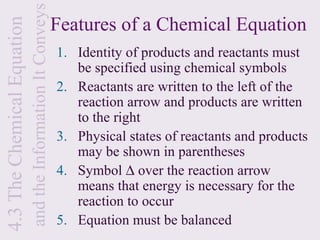
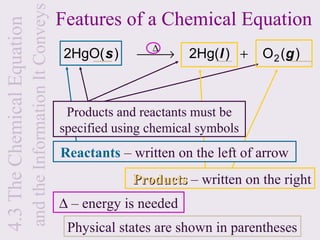
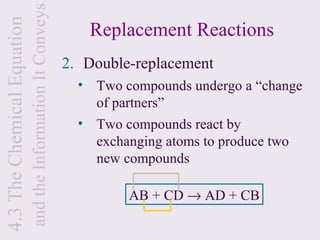
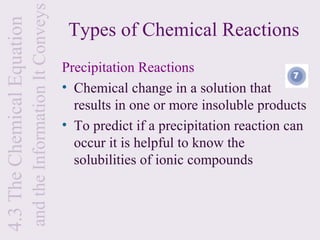
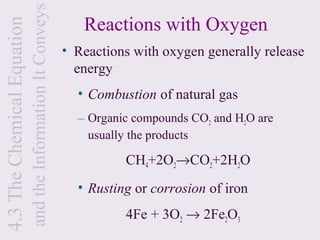
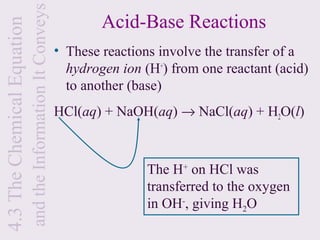
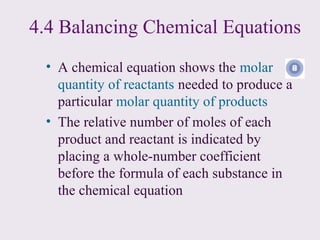
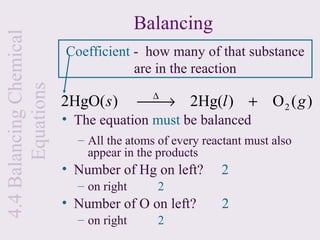
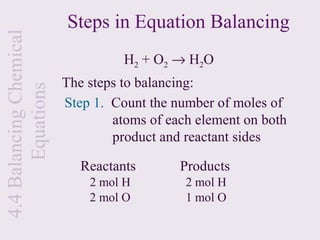
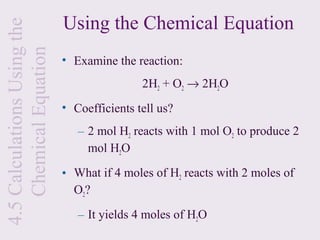
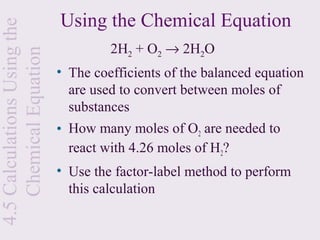
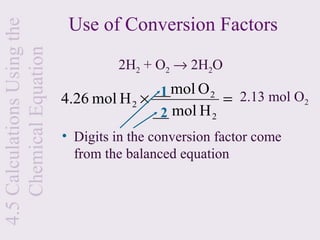
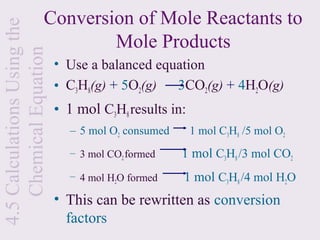
3) Chemical equations are used to represent chemical reactions and must satisfy the law of conservation of mass by being balanced with the same number and type of atoms on each side of the reaction arrow.




























![4.5 Calculations Using the Relating Masses of Reactants
Chemical Equation and Products
• Calculate grams C3H8 required to produce
36.0 grams of H2O
• Use 3 conversion factors
– Grams H2O to moles H2O
– Moles H2O to moles C3H8
– Moles of C3H8 to grams C3H8
grams moles moles grams
H2 O H2 O C3 H8 C3 H8
– Set up the equation and cancel units
36.0 g H2O x 1 mol H2O x 1 mol C3H8 x 44.0 g C3H8
18.0 g H2O 4 mol H2O 1 mol C3H8
– 36.0 x [1/18.0] x [1/4] x 44.0 g C3H8 = 22.0 g C3H8](https://image.slidesharecdn.com/mecchapter4-120815081521-phpapp02/85/Me-cchapter-4-59-320.jpg)
![Calculating a Quantity of Reactant
4.5 Calculations Using the
• Ca(OH)2 neutralizes HCl
Chemical Equation
• Calculate grams HCl neutralized by 0.500 mol
Ca(OH)2
– Write chemical equation and balance
• Ca(OH)2(s) + 2HCl(aq) CaCl2(s) + 2H2O(l)
– Plan the path
moles moles grams
Ca(OH)2 HCl HCl
– Set up the equation and cancel units
0.500 mol Ca(OH)2 x 2 mol HCl x 36.5 g HCl
1 mol Ca(OH)2 1 mol HCl
Solve equation 0.500 x [2/1] x 36.5 g HCl = 36.5 g HCl](https://image.slidesharecdn.com/mecchapter4-120815081521-phpapp02/85/Me-cchapter-4-60-320.jpg)
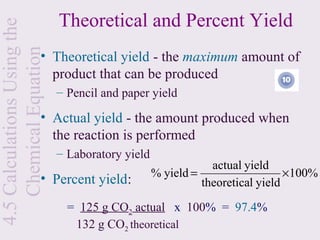
![Sample Calculation
4.5 Calculations Using the
If the theoretical yield of iron was 30.0 g
Chemical Equation
and actual yield was 25.0 g, calculate the
percent yield:
2 Al(s) + Fe2O3(s) → Al2O3(aq) + 2Fe(aq)
• [25.0 g / 30.0 g] x 100% = 83.3%
• Calculate the % yield if 26.8 grams iron
was collected in the same reaction](https://image.slidesharecdn.com/mecchapter4-120815081521-phpapp02/85/Me-cchapter-4-64-320.jpg)