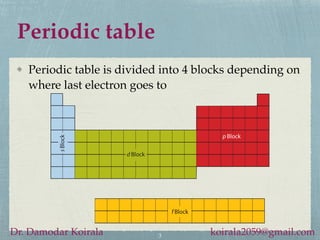
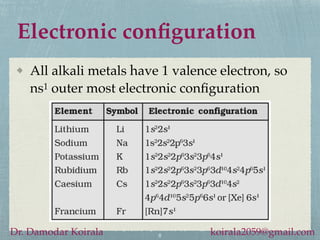
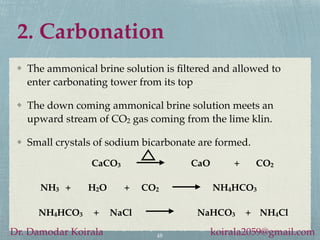
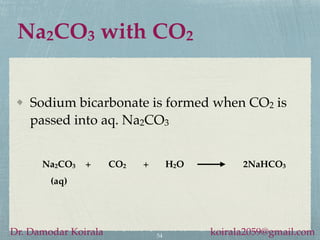
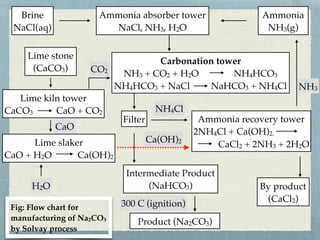
This document provides information about alkali metals and describes the extraction and uses of sodium and sodium compounds. It discusses the periodic table classification of alkali metals. Key points include:
- Alkali metals are soft, silvery-white reactive metals found in Group 1 of the periodic table.
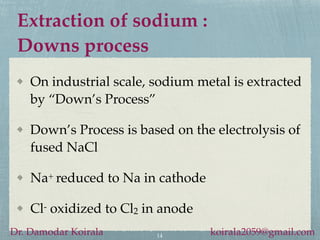
- Sodium is extracted commercially via the Downs process, which involves electrolysis of molten sodium chloride.
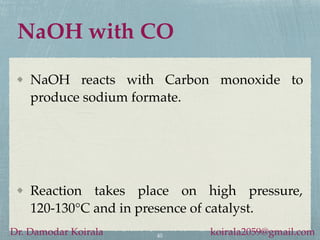
- Sodium reacts violently with water and is used to produce sodium hydroxide and other sodium compounds.
- Sodium hydroxide and sodium carbonate are manufactured via electrolysis or other chemical processes and have various industrial and household applications.