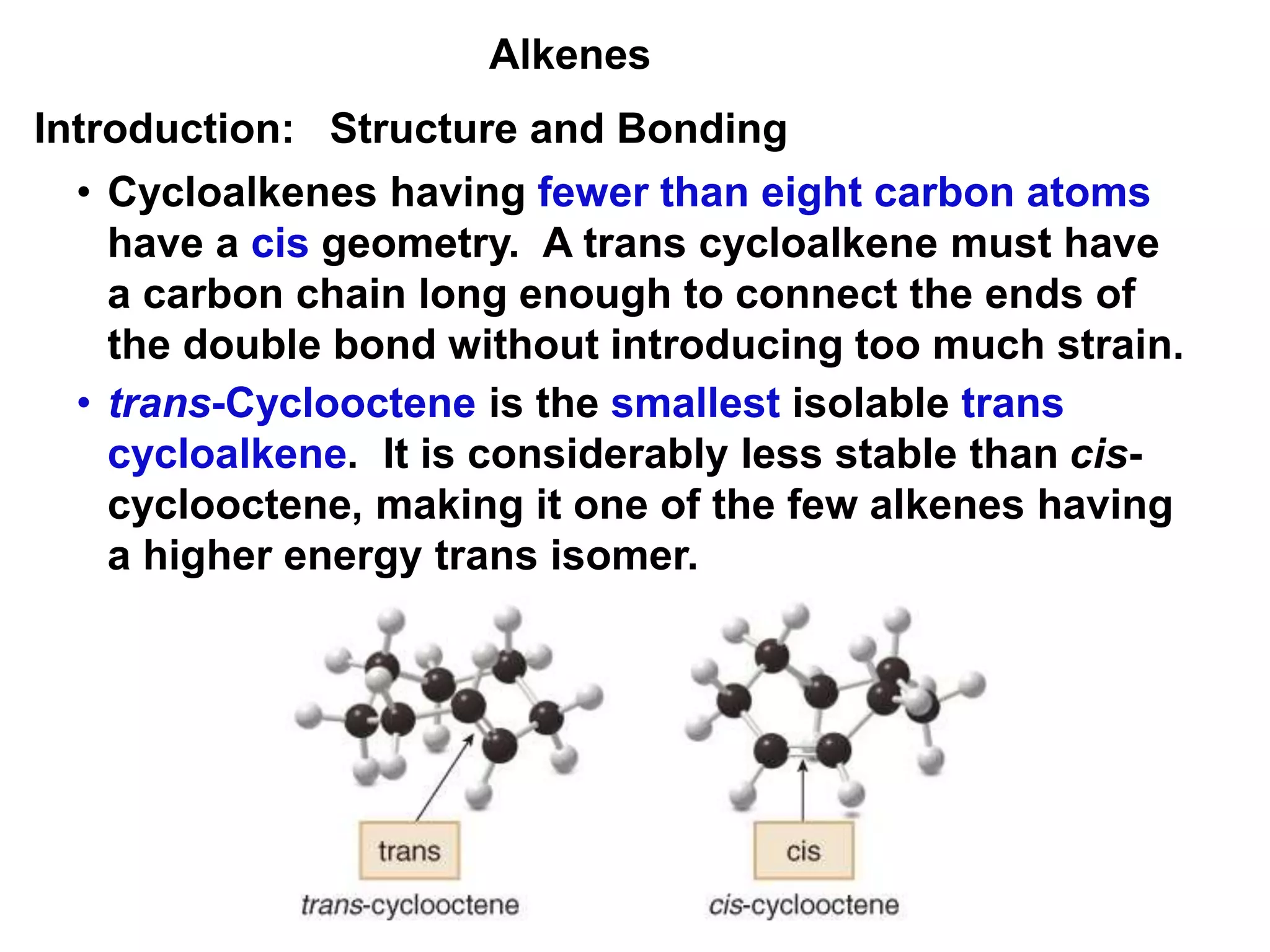
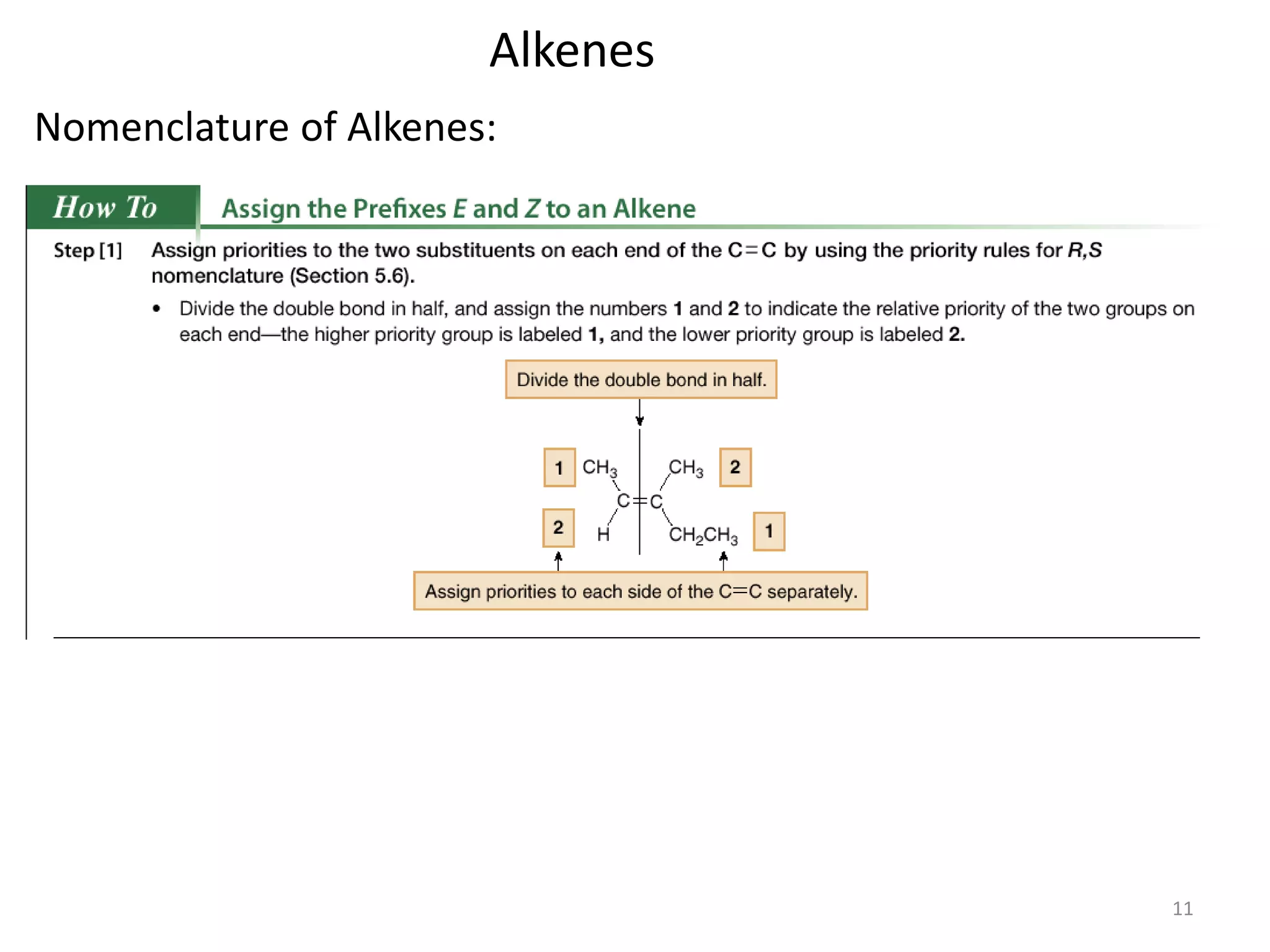
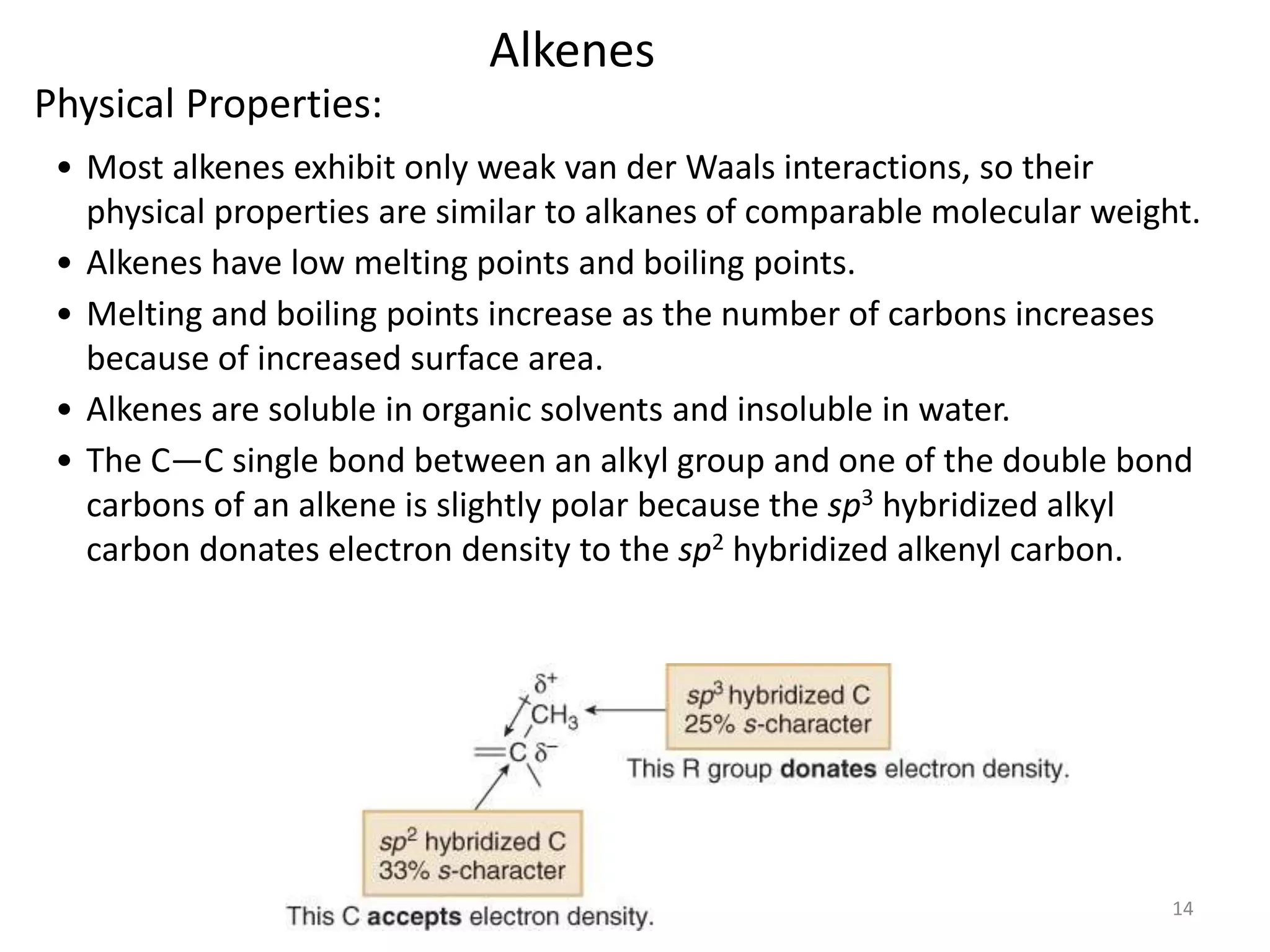
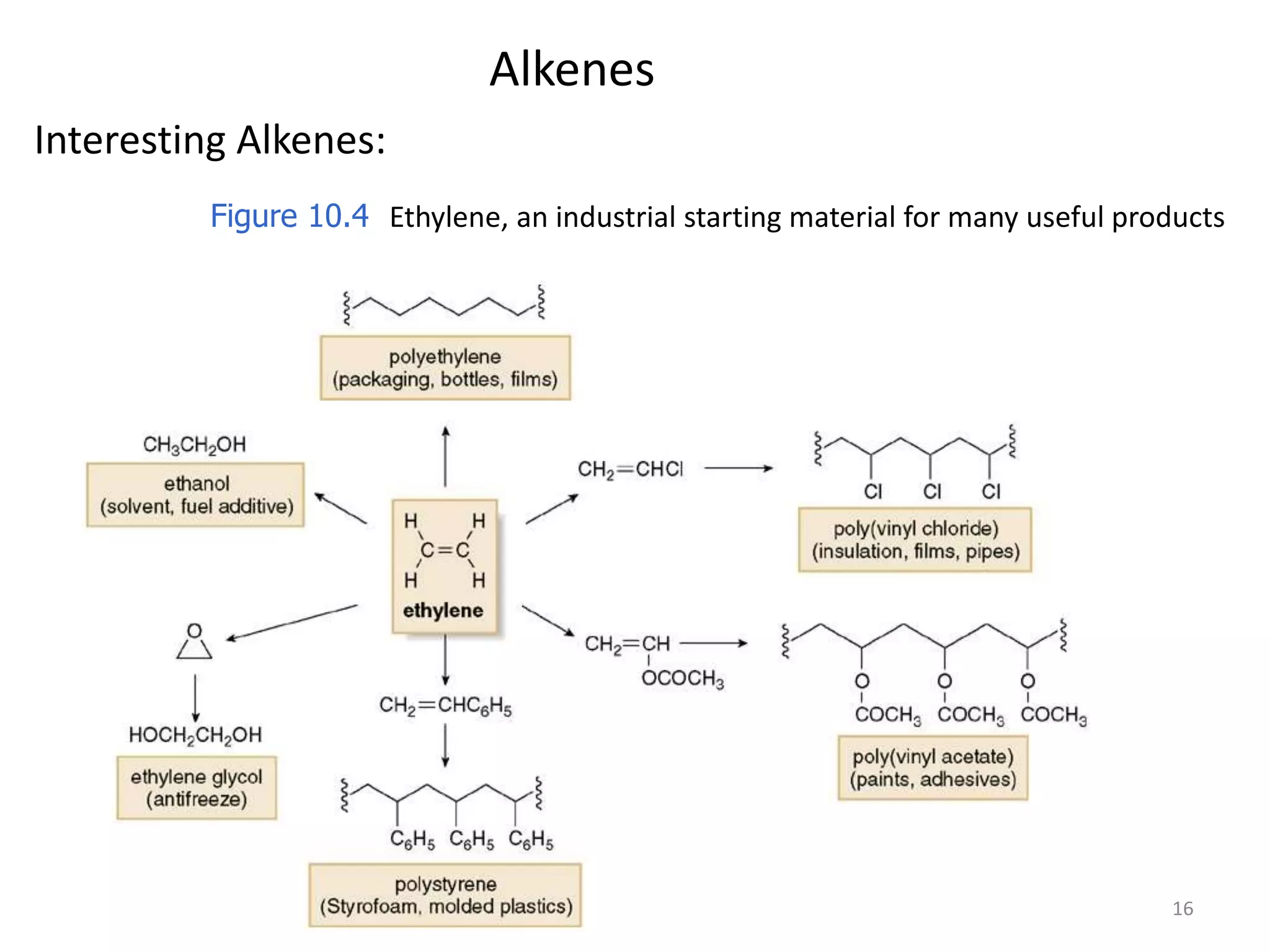
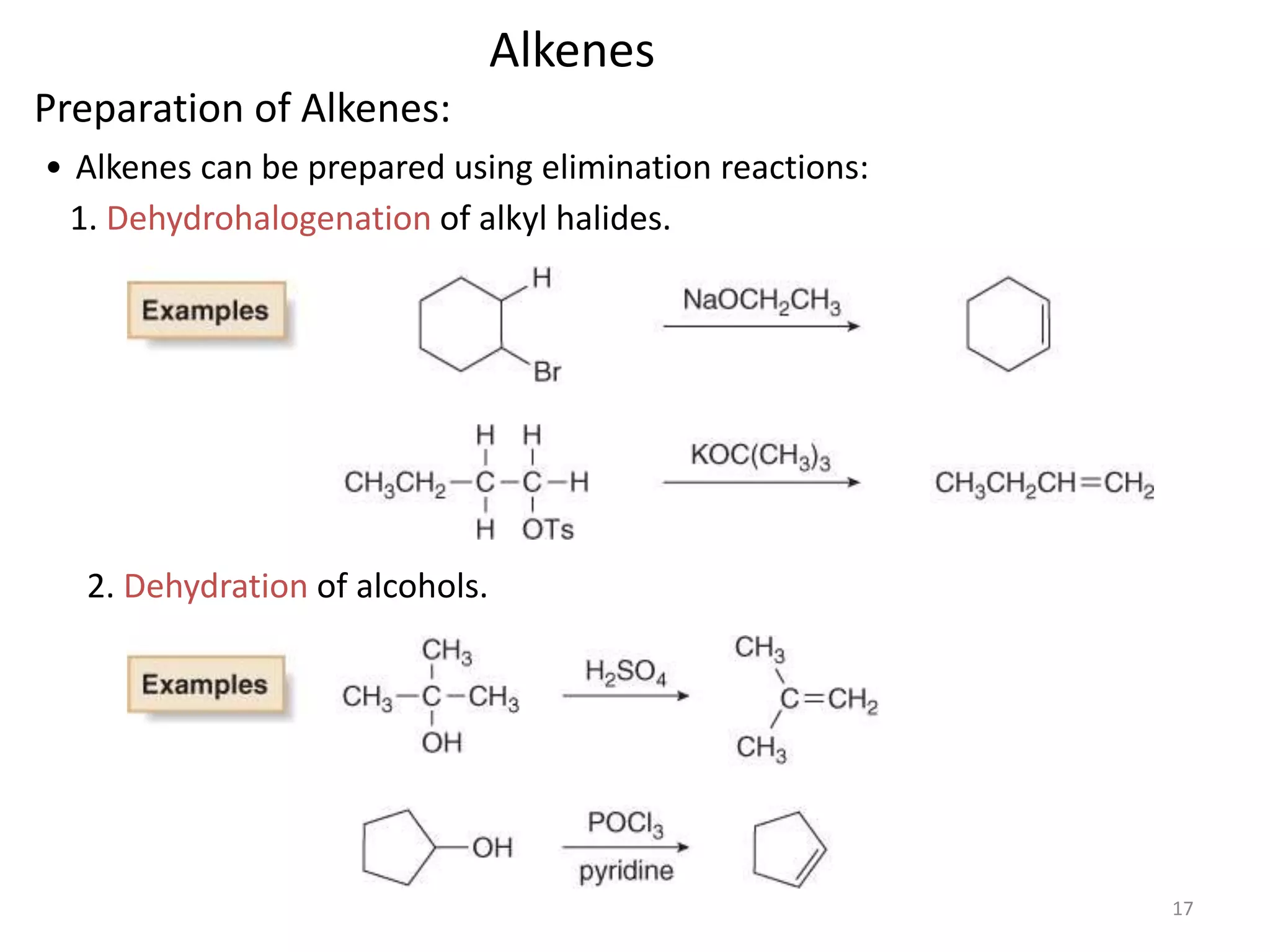
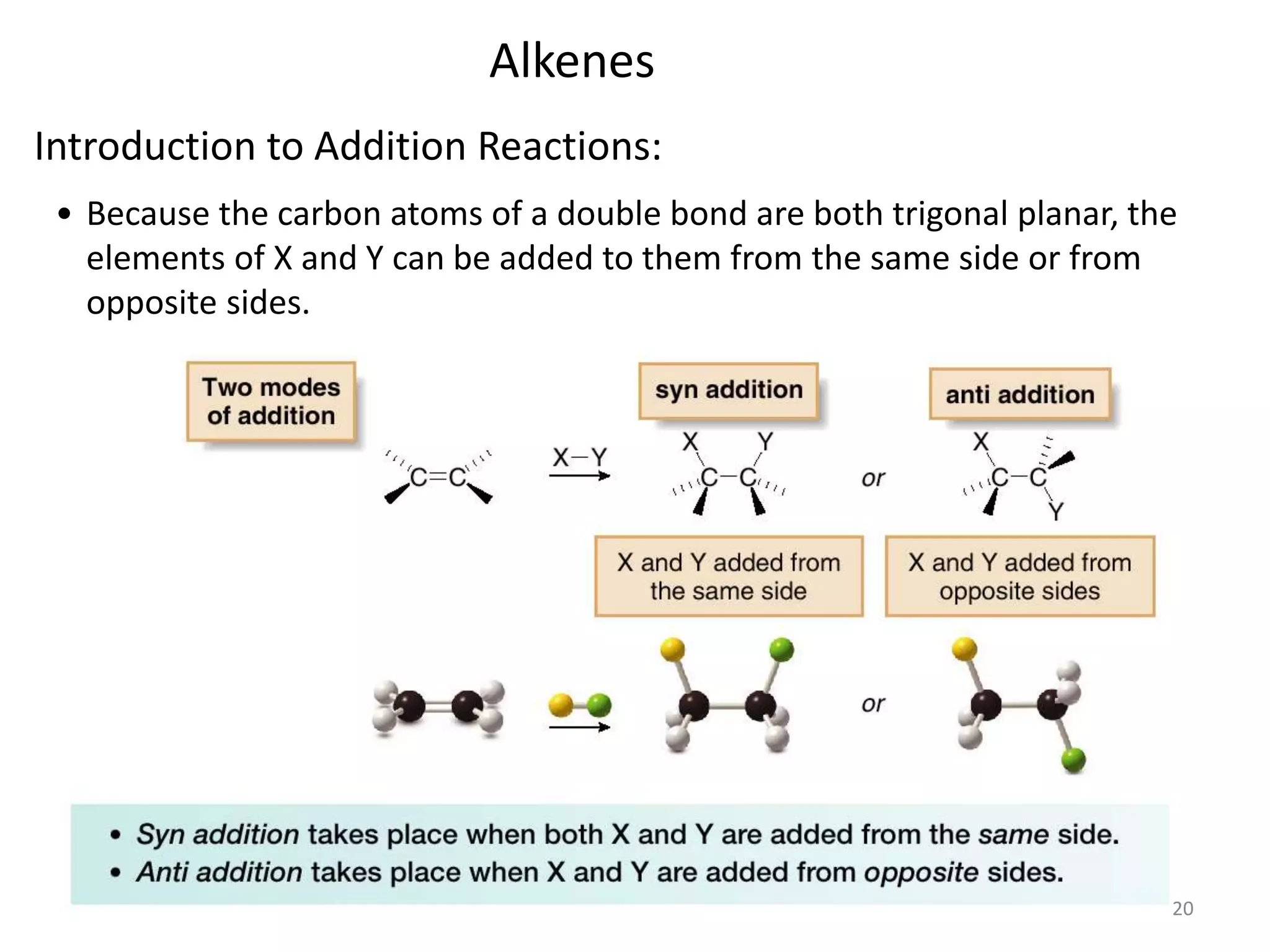
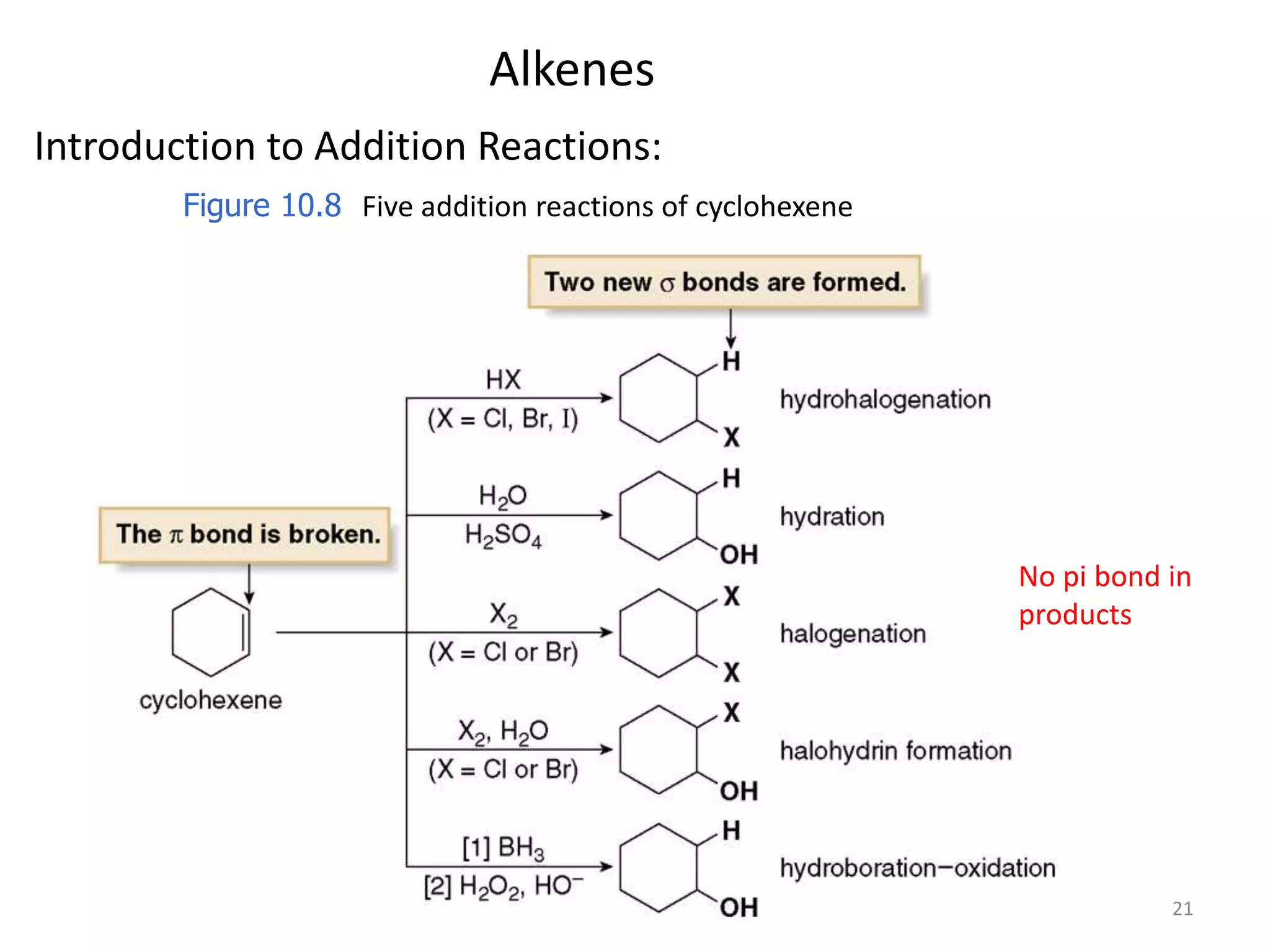
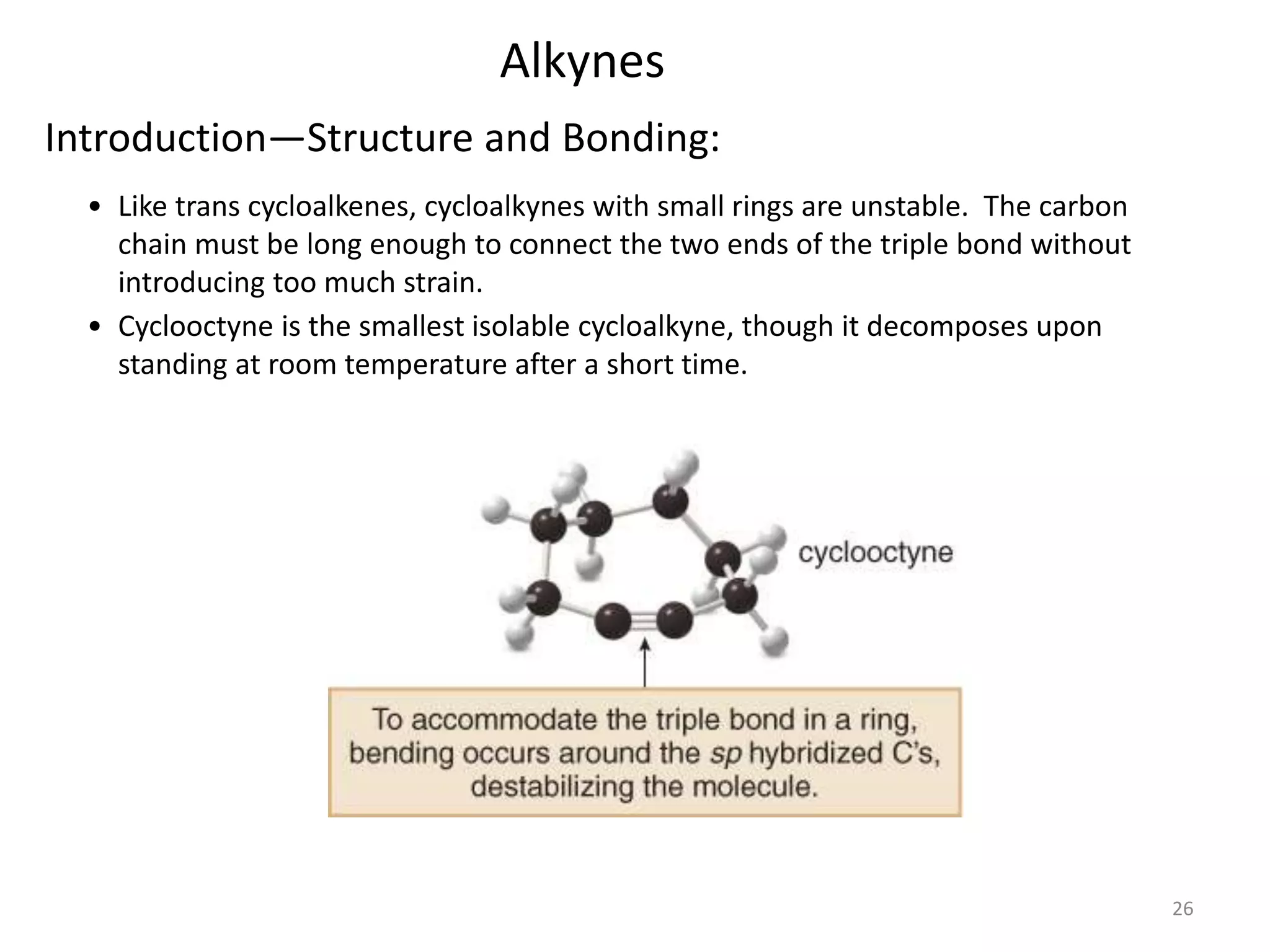
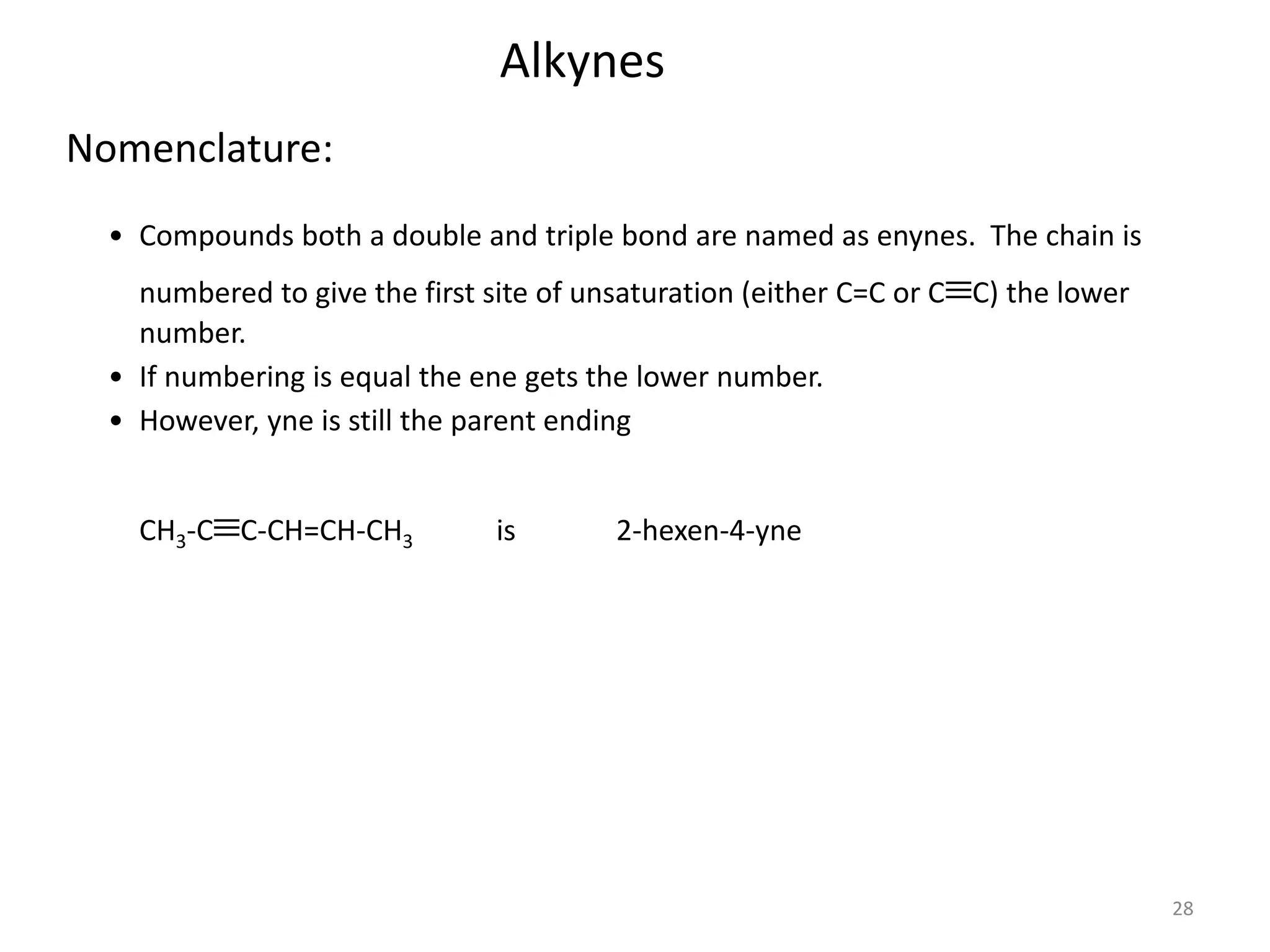
This document provides an introduction to organic chemistry concepts related to alkenes and alkynes. It discusses the structure and bonding of alkenes and alkynes, including sp2 and sp3 hybridization. It also covers nomenclature rules for naming alkenes and alkynes according to IUPAC conventions. In addition, it discusses the preparation of alkenes and alkynes through elimination reactions like dehydrohalogenation and dehydration, as well as common physical properties.