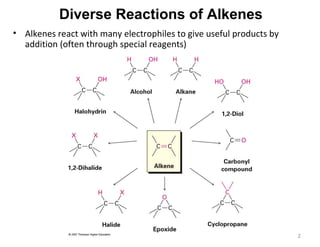
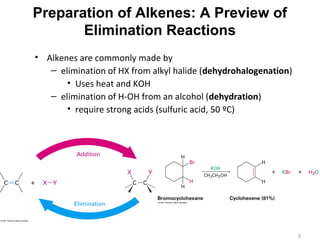
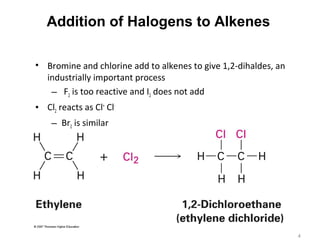
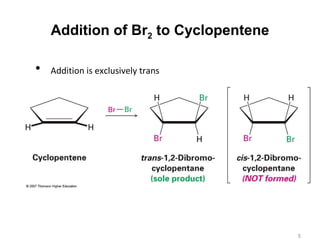
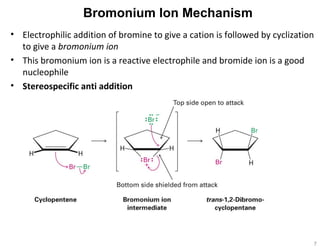
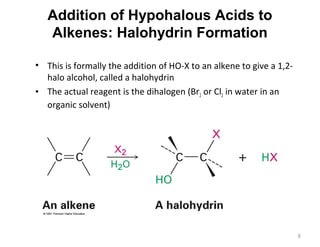
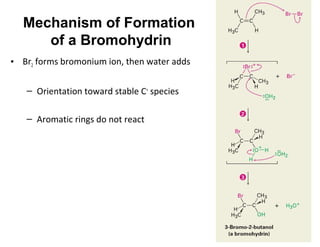
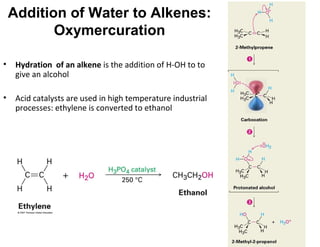
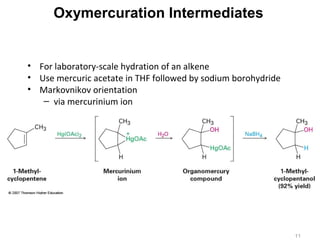
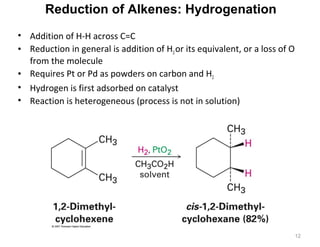
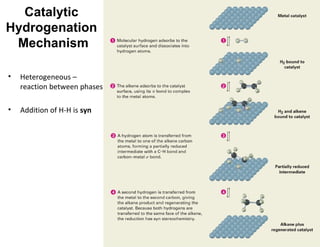
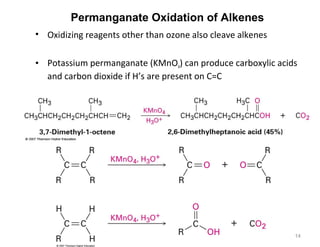
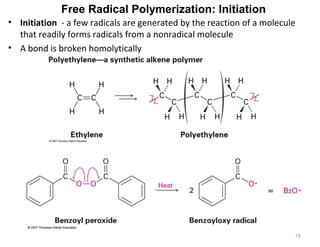
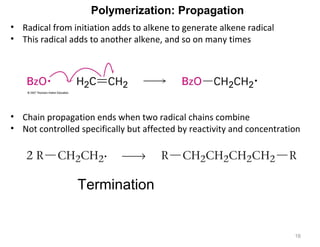
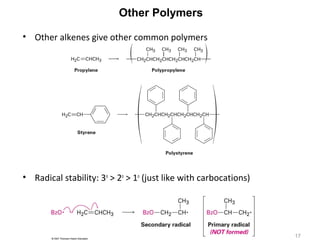
The document discusses the reactions and synthesis of alkenes, highlighting their reactivity with electrophiles and methods of preparation through elimination reactions. It details mechanisms for the addition of halogens and hypohalous acids, oxymercuration, and reduction processes such as hydrogenation. Additionally, the document covers oxidation using potassium permanganate and the principles of free radical polymerization, including initiation, propagation, and termination.