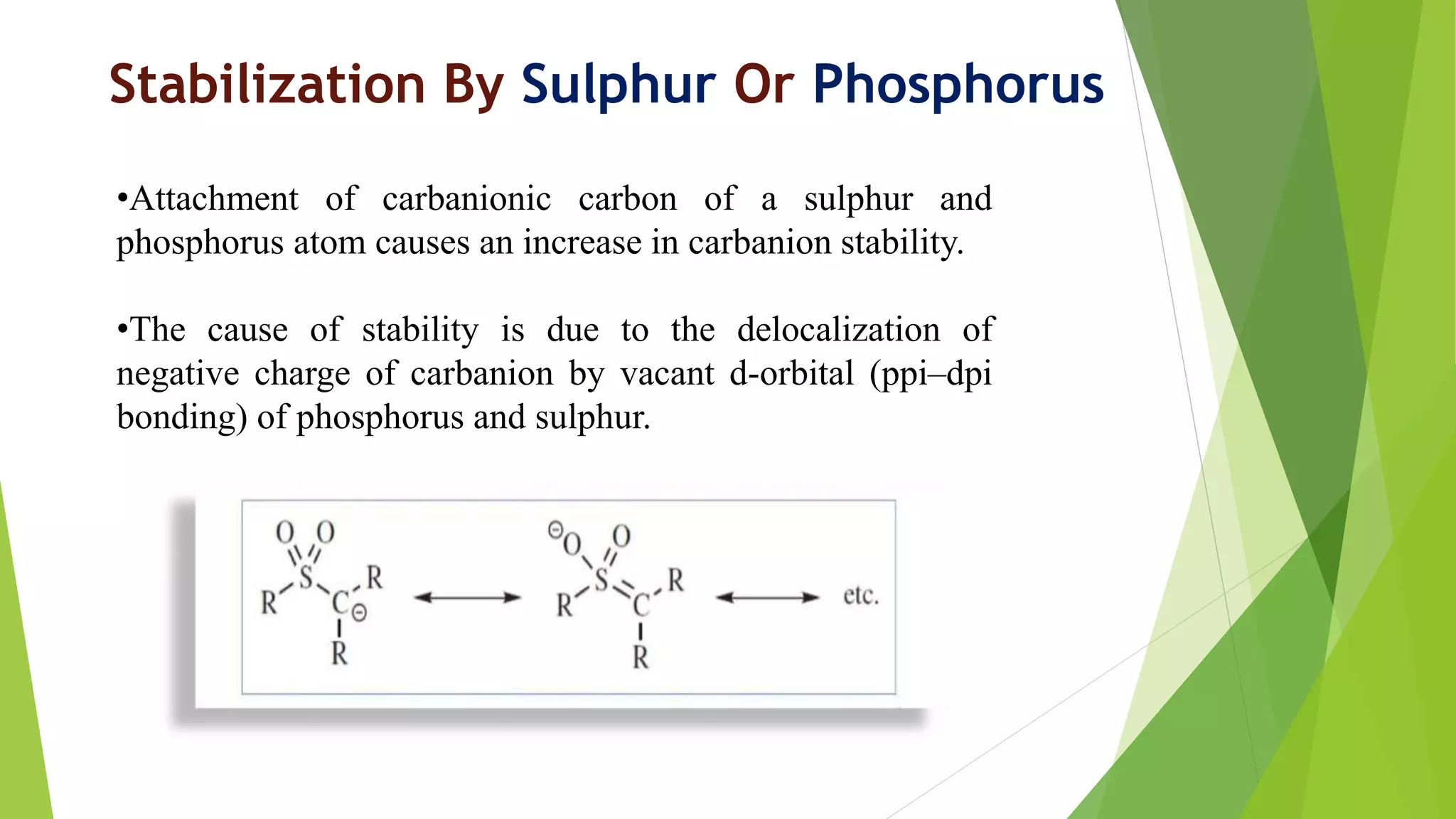
This document discusses carbanions, which are negatively charged organic species where carbon carries three bond pairs and one lone pair. Carbanions are stabilized through conjugation, resonance effects, field effects, and aromaticity. They are generated through heterolytic bond cleavage or addition of a negative ion to a carbon-carbon multiple bond. As nucleophiles, carbanions undergo reactions such as alpha-halogenation of ketones, additions to carbonyls, nucleophilic acyl substitutions, substitutions with alkyl halides, and Michael additions.