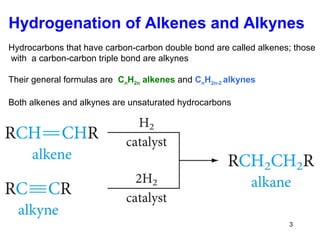
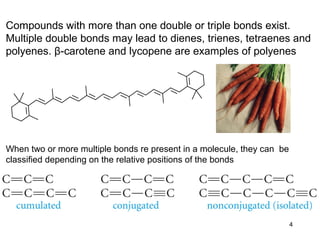
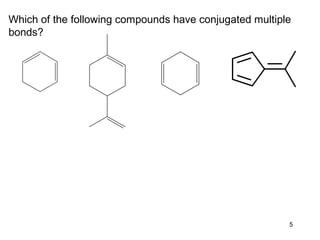
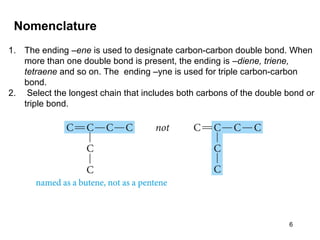
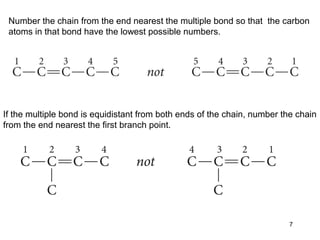
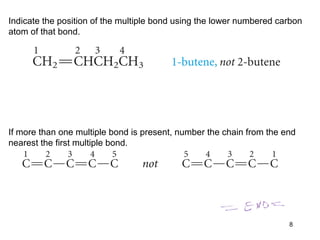
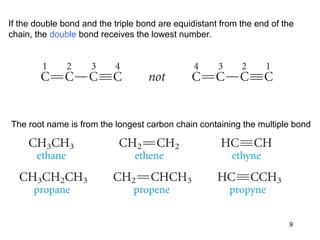
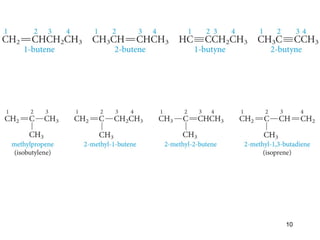
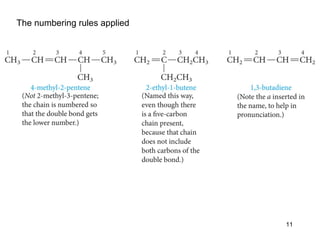
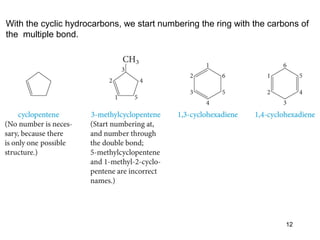
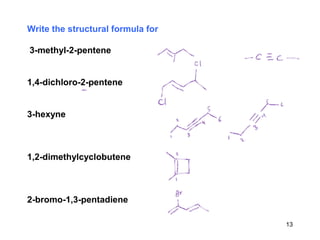
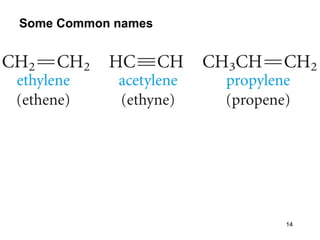
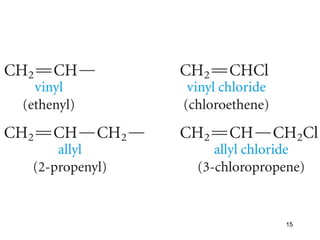
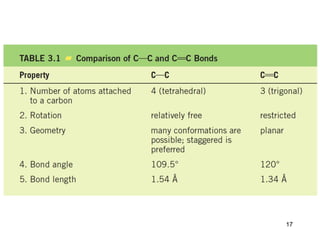
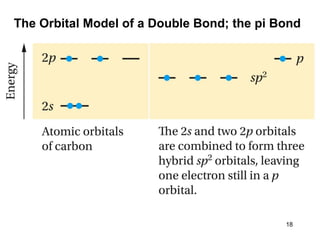
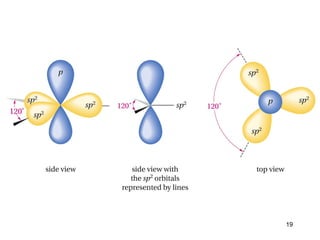
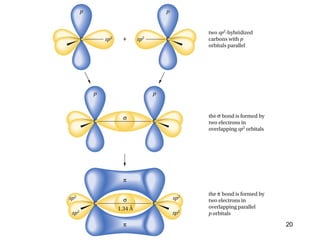
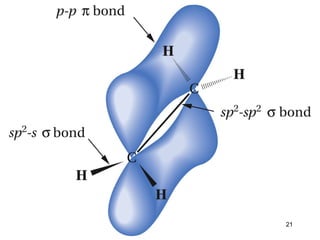
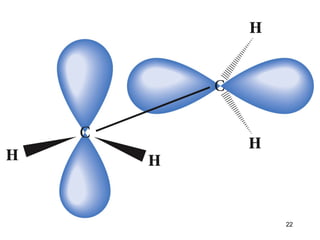
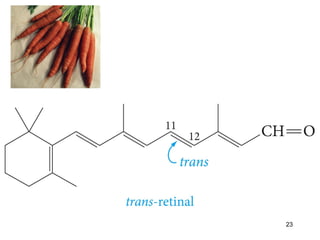
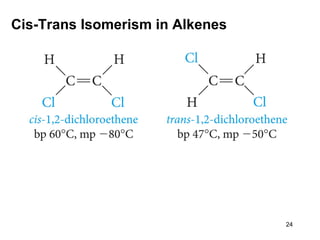
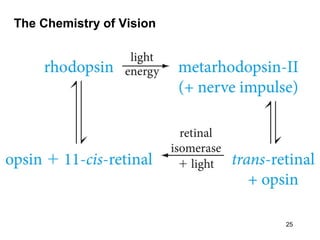
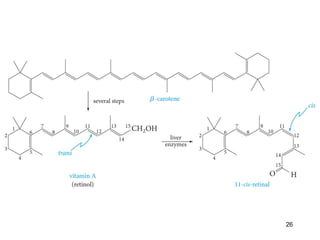
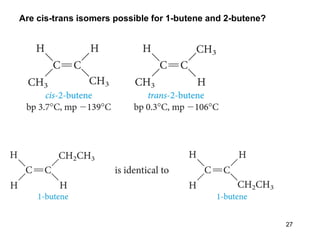
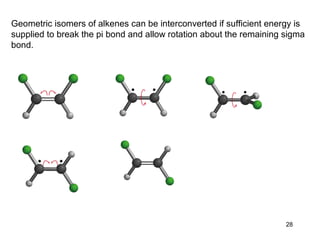
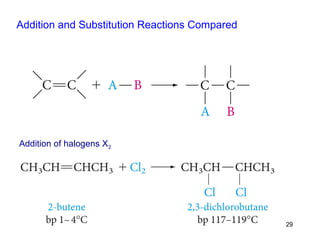
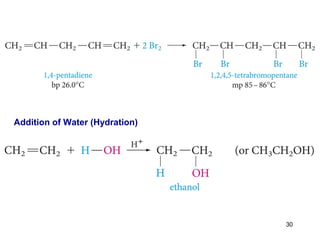
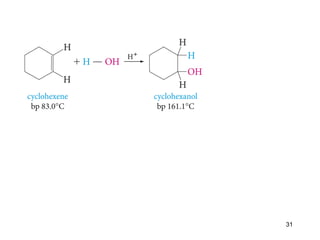
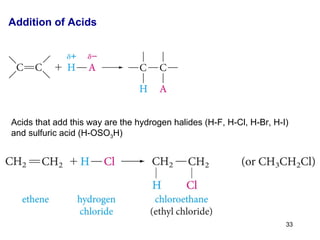
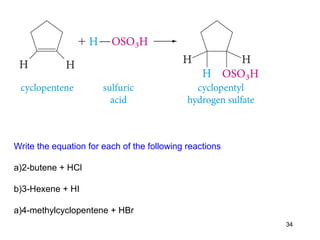
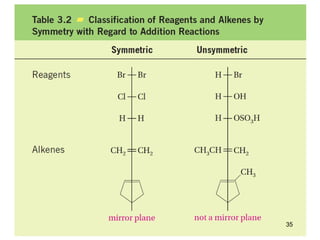
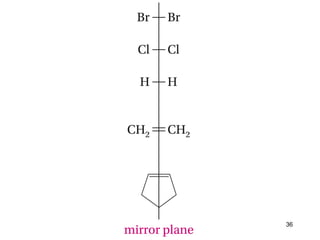
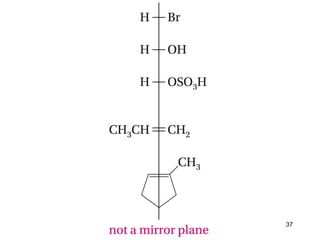
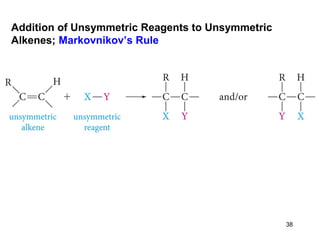
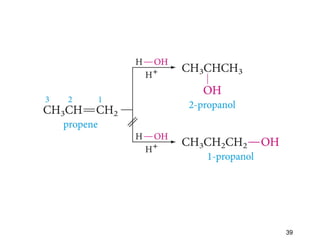
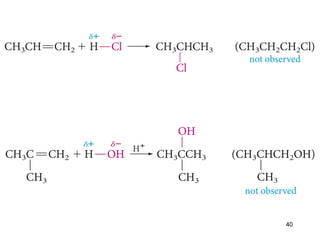
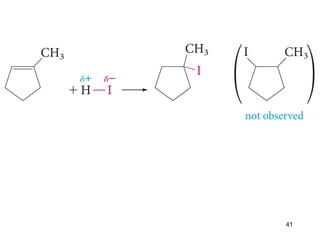
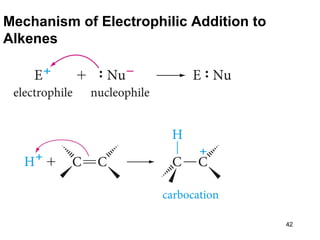
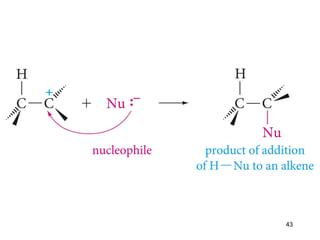
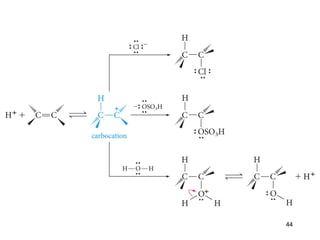
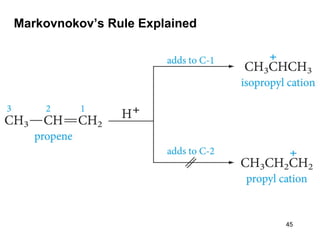
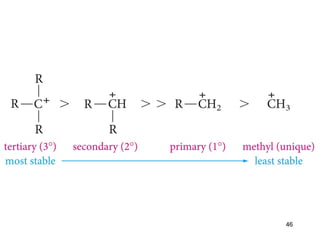
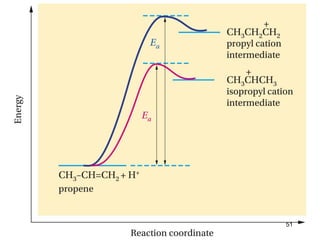
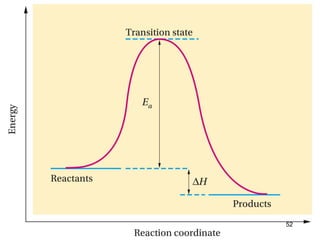
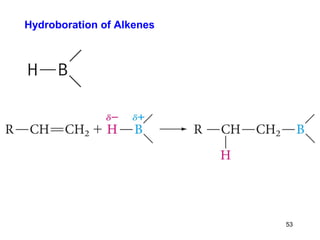
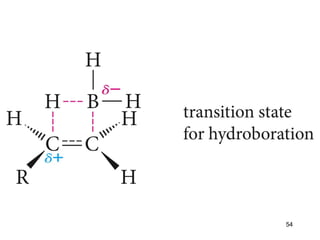
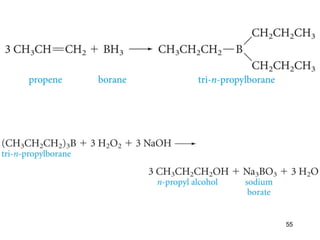
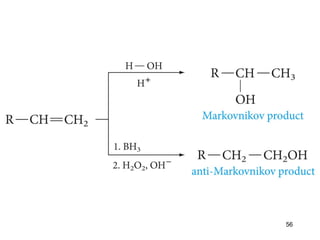
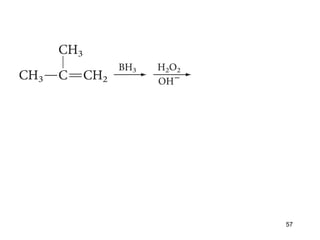
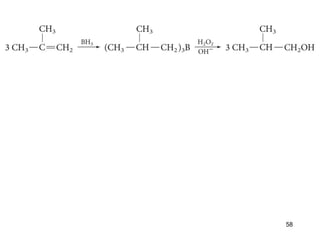
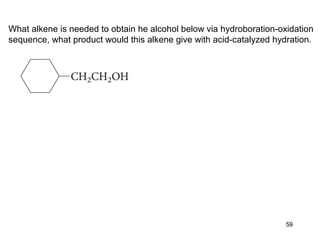
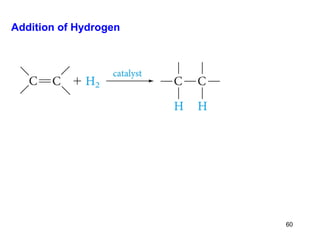
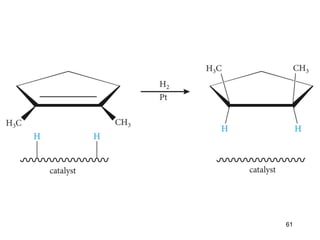
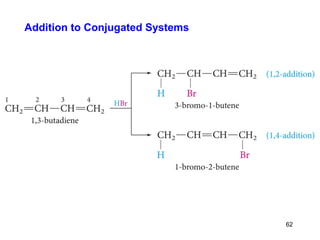
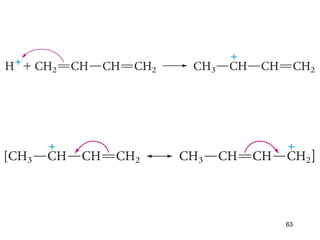
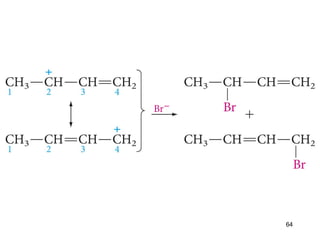
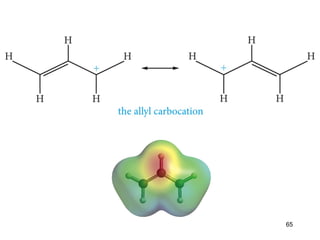
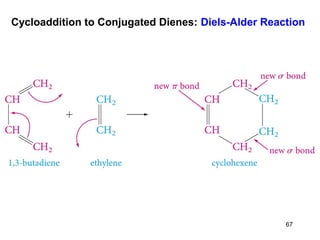
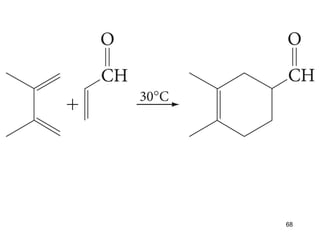
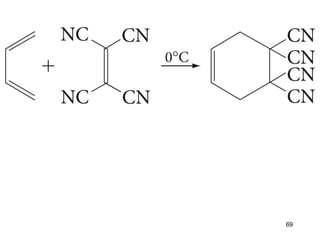
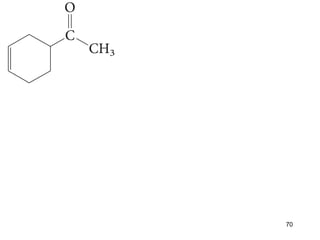
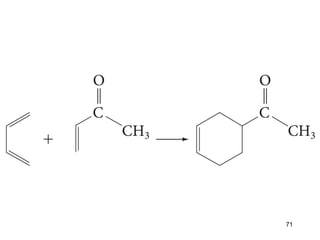
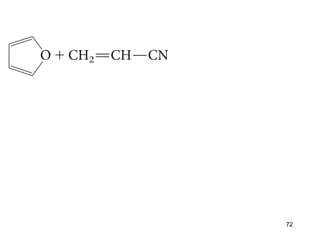
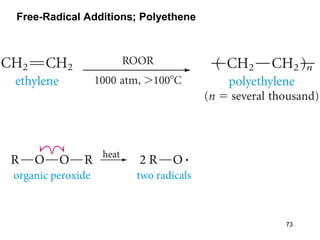
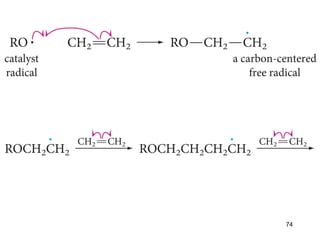
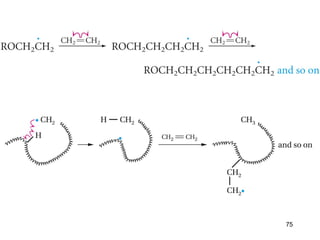
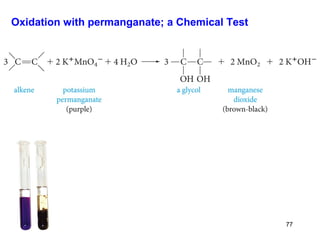
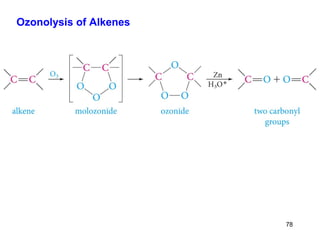
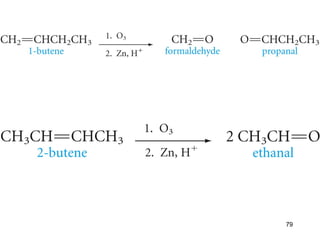
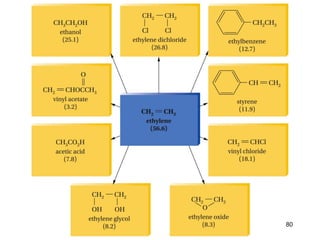
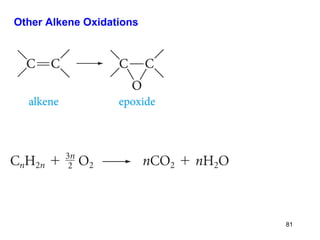
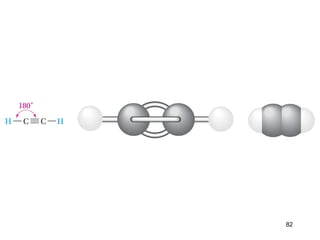
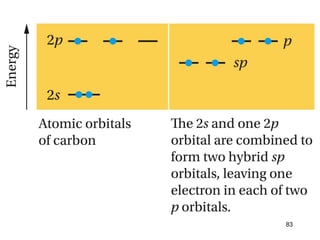
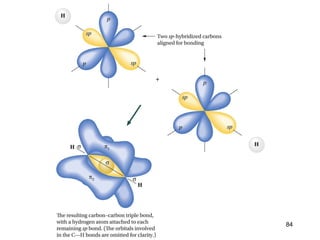
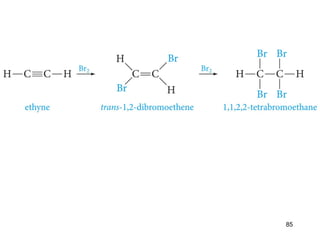
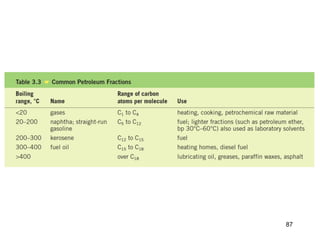
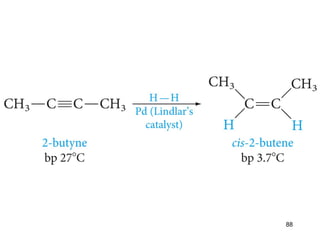
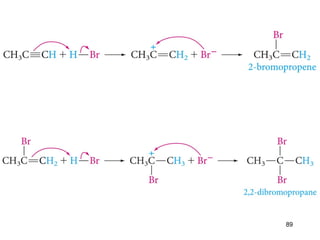
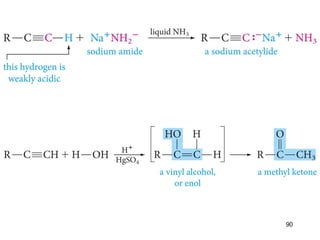
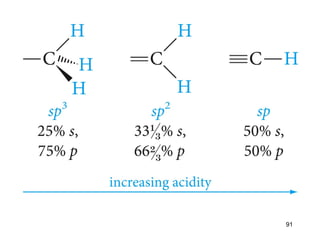
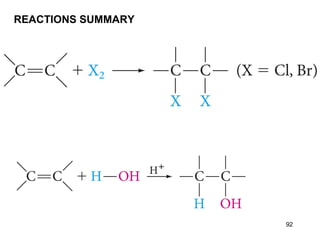
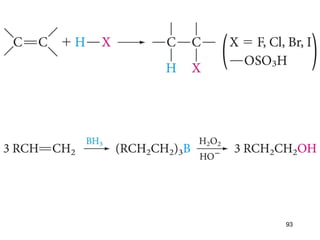
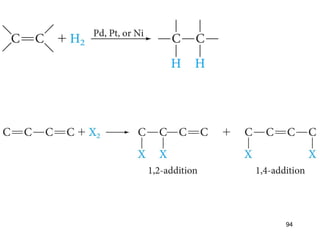
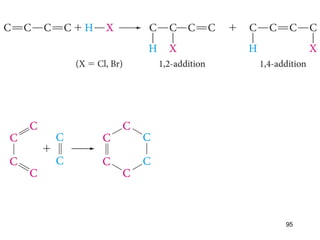
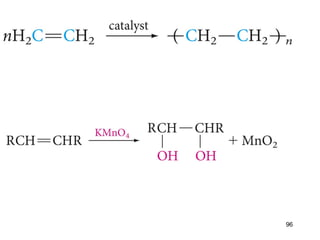
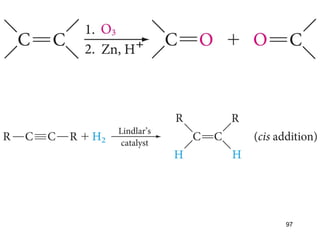
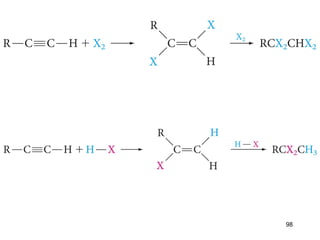
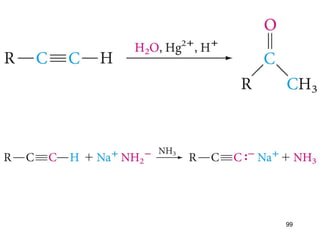
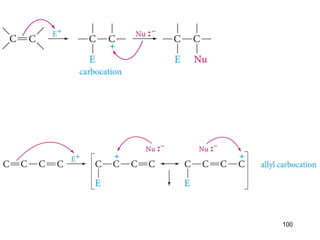
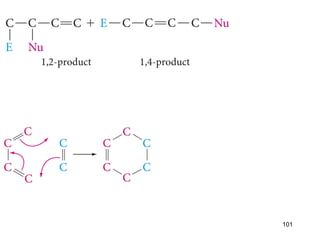
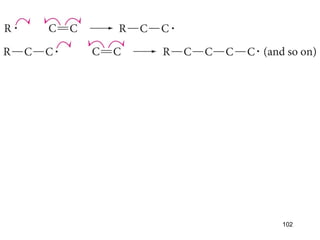
The document discusses different types of reactions that alkenes and alkynes undergo. It describes how alkenes contain carbon-carbon double bonds and alkynes contain carbon-carbon triple bonds. Some key reactions mentioned include hydroboration-oxidation of alkenes, hydration of alkenes using acid catalysts, electrophilic addition reactions of alkenes that follow Markovnikov's rule, and the Diels-Alder reaction between conjugated dienes. Oxidation reactions of alkenes involving permanganate and ozonolysis are also summarized.