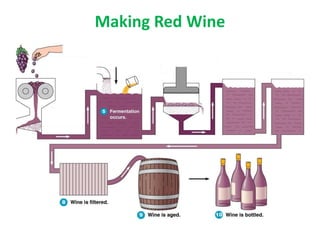
The document discusses the microbiological spoilage and preservation of pharmaceutical products, focusing on food categories based on spoilage likelihood and various food-processing methods such as canning, pasteurization, and irradiation. It highlights the risks associated with microbial contamination in food, including pathogens like Salmonella and E. coli, and emphasizes the importance of proper handling and storage. Additionally, it details methods for food preservation, including lyophilization and fermentation, while addressing the need for quality control in food safety.