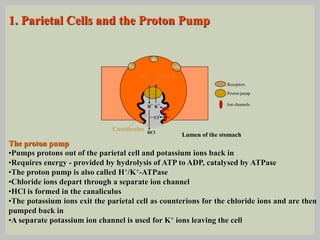
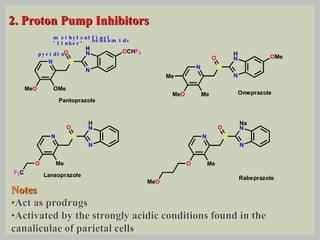
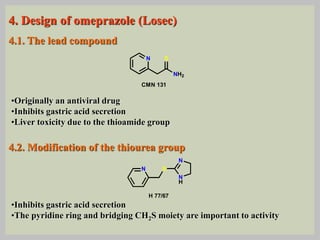
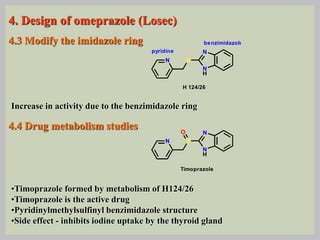
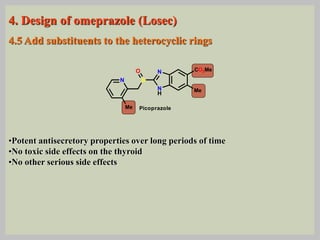
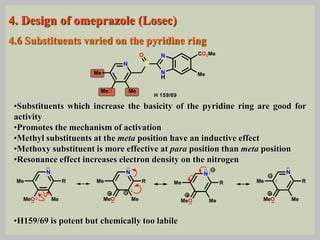
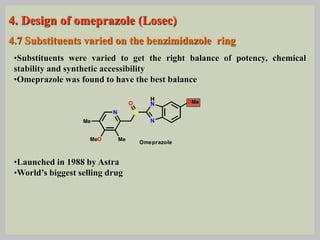
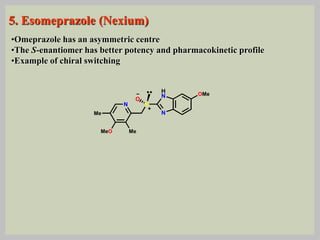
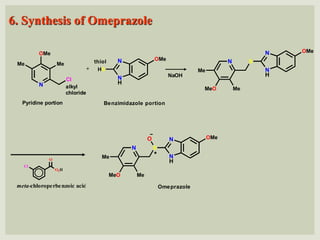
The document discusses proton pump inhibitors (PPIs), focusing on their mechanism of action, specifically the proton pump located in parietal cells. It details the drug design and structure of omeprazole, its modifications, and also highlights the advantages of its s-enantiomer, esomeprazole. Additionally, the document covers the synthesis process of omeprazole and its significance in inhibiting gastric acid secretion.