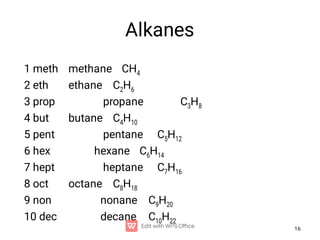
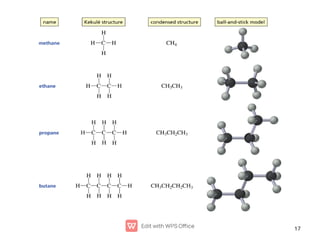
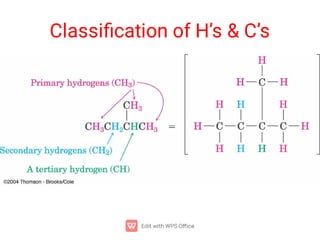
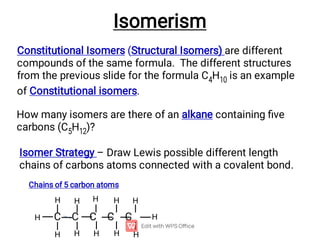
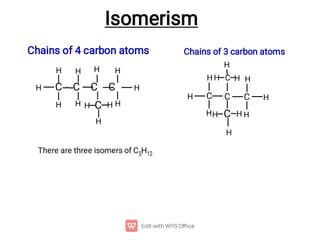
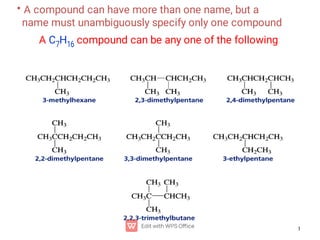
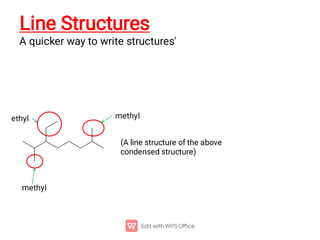
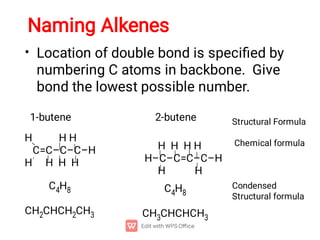
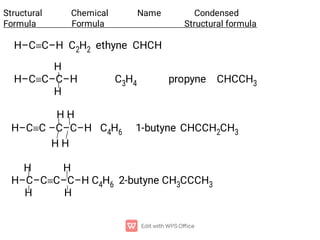
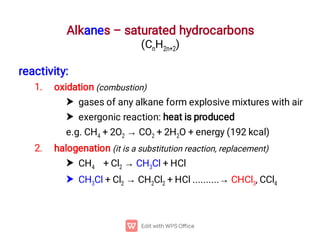
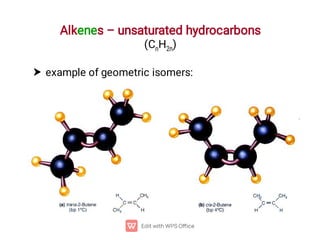
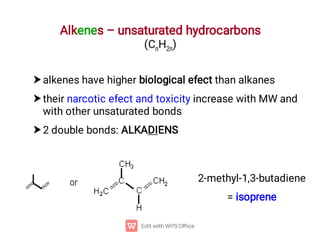
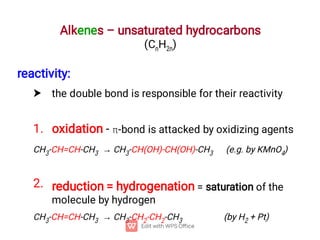
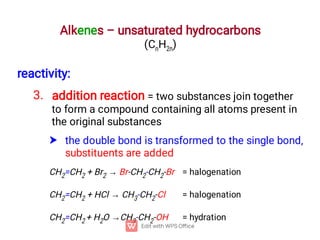
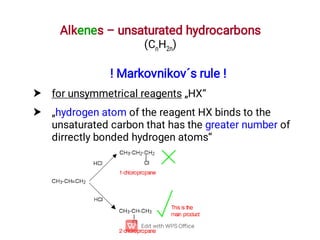
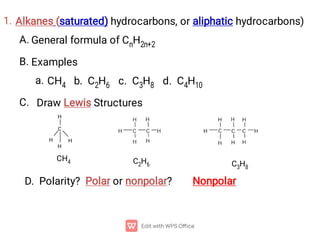
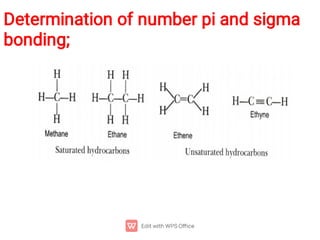
Organic chemistry is the study of carbon compounds. Hydrocarbons are organic compounds composed entirely of carbon and hydrogen. There are two main types of hydrocarbons: aliphatic and aromatic. Aliphatic hydrocarbons can be classified as alkanes, alkenes, alkynes, or cycloalkanes depending on the presence of single, double, or triple carbon bonds. Alkanes contain only single bonds and follow the general formula CnH2n+2. Common reactions of alkanes include combustion and halogenation. Alkenes contain double bonds and have the formula CnH2n. They exhibit geometric isomerism and undergo addition reactions. Alkynes have triple bonds and the






![15
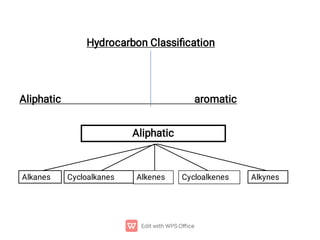
Hydrocarbons
Alkanes contain only single ( ) bonds and have the generic
molecular formula: [CnH2n+2]
Alkenes also contain double ( + ) bonds and have the generic
molecular formula: [CnH2n]
Alkynes contain triple ( + 2) bonds and have the generic
molecular formula: [CnH2n-2]
Aromatics are planar, ring structures with alternating single and
double bonds: eg. C6H6](https://image.slidesharecdn.com/theoryorganic1-231006170405-562fc4ff/85/theory-organic-1-pdf-15-320.jpg)