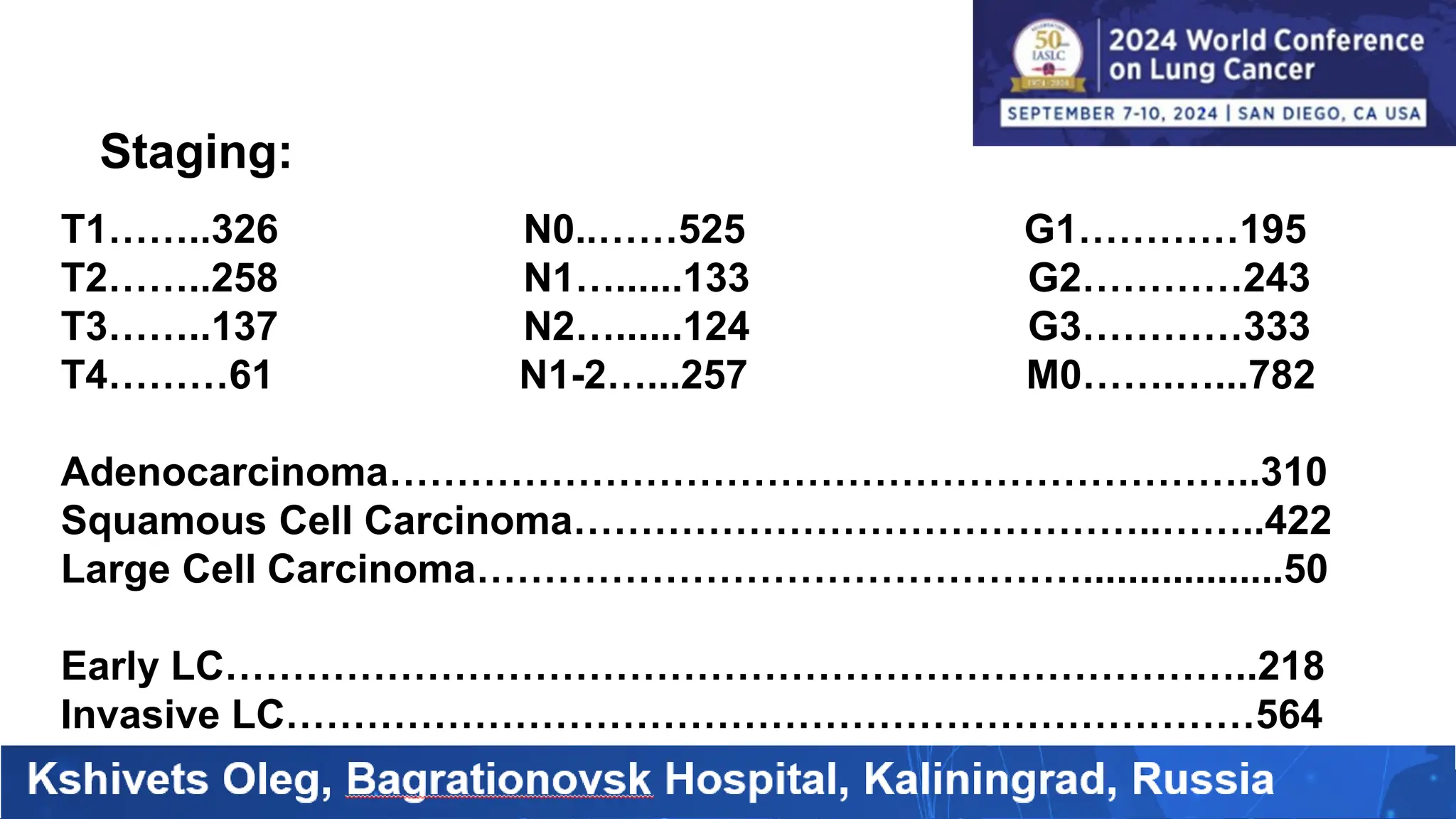
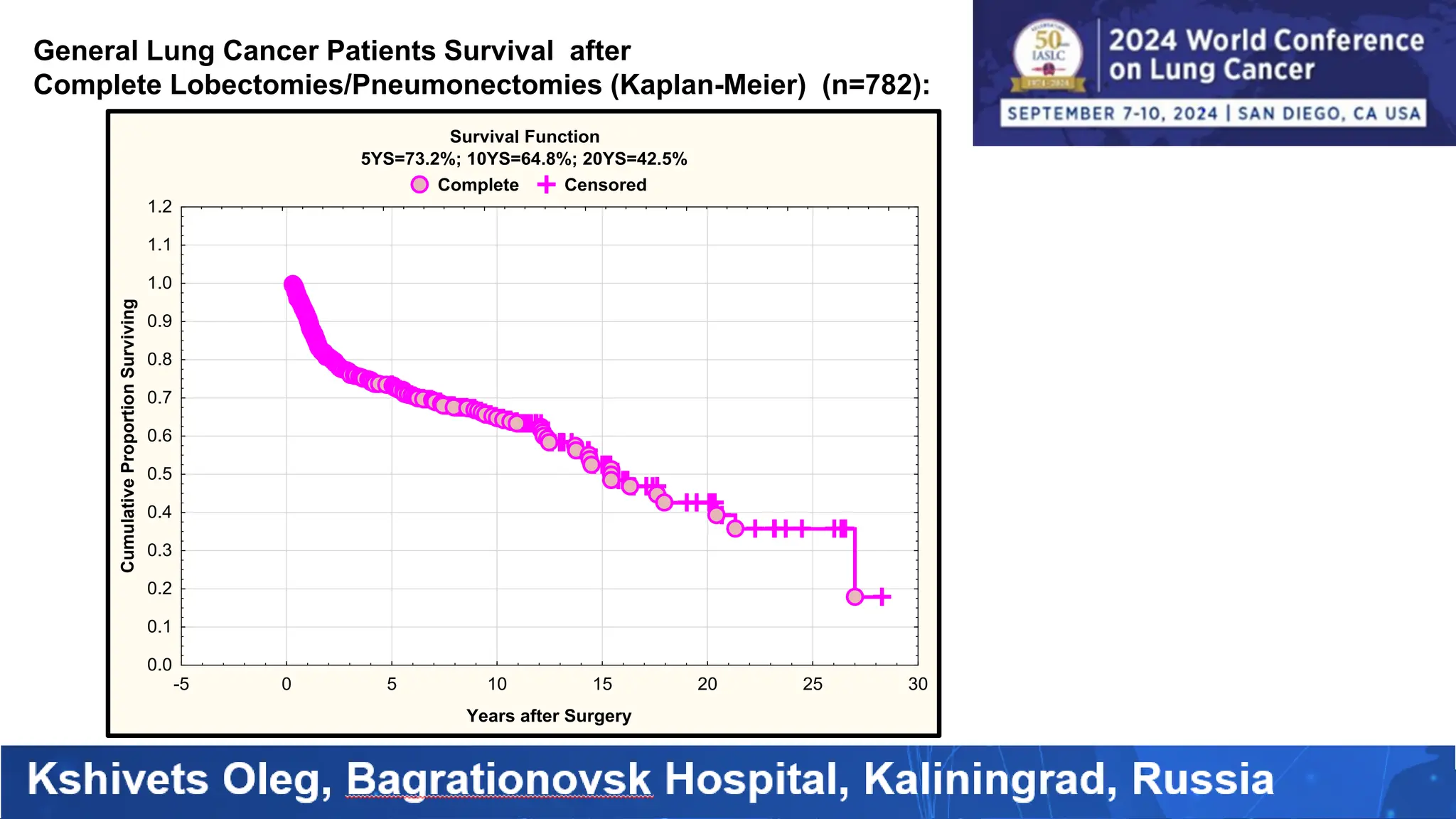
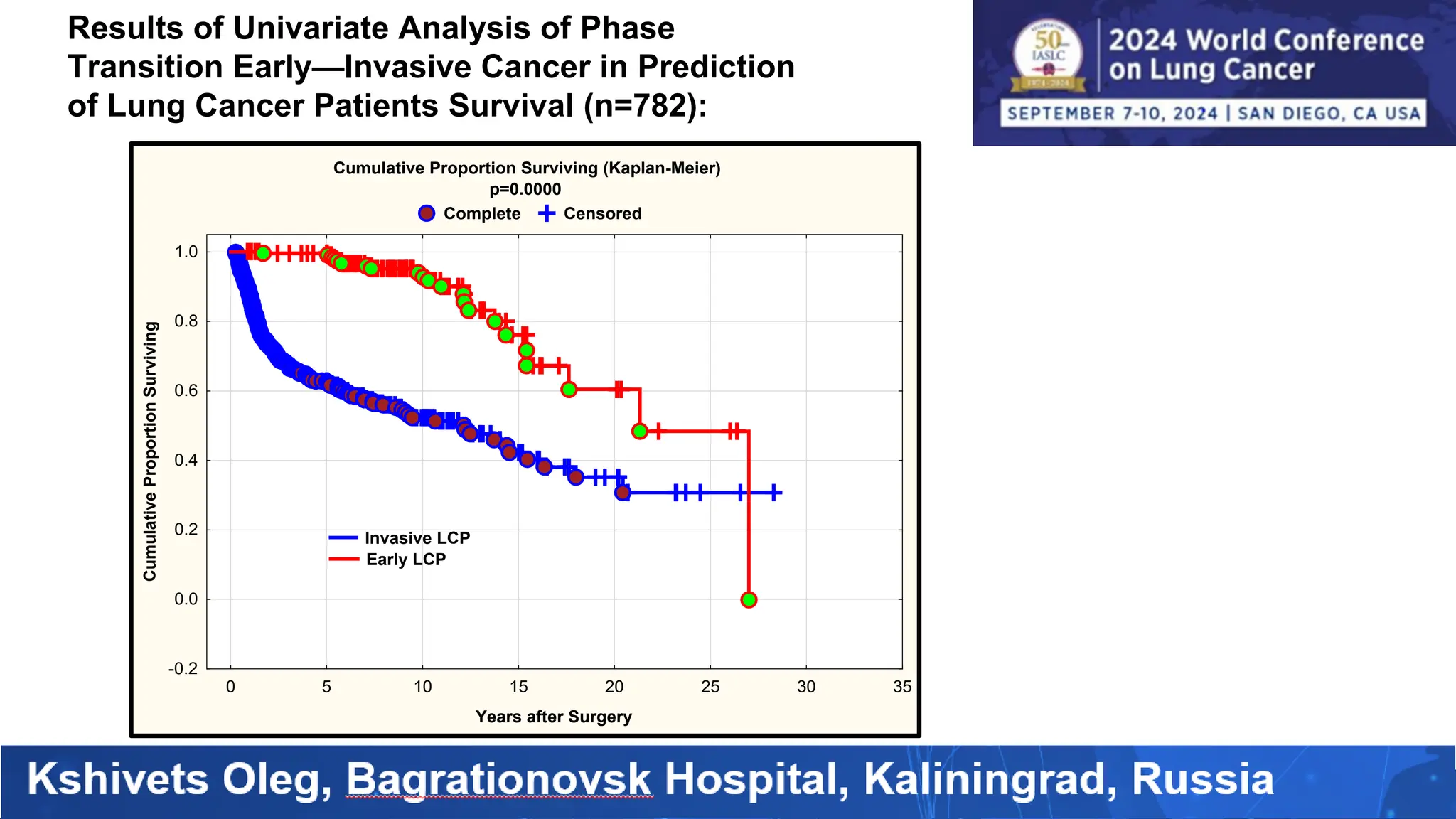
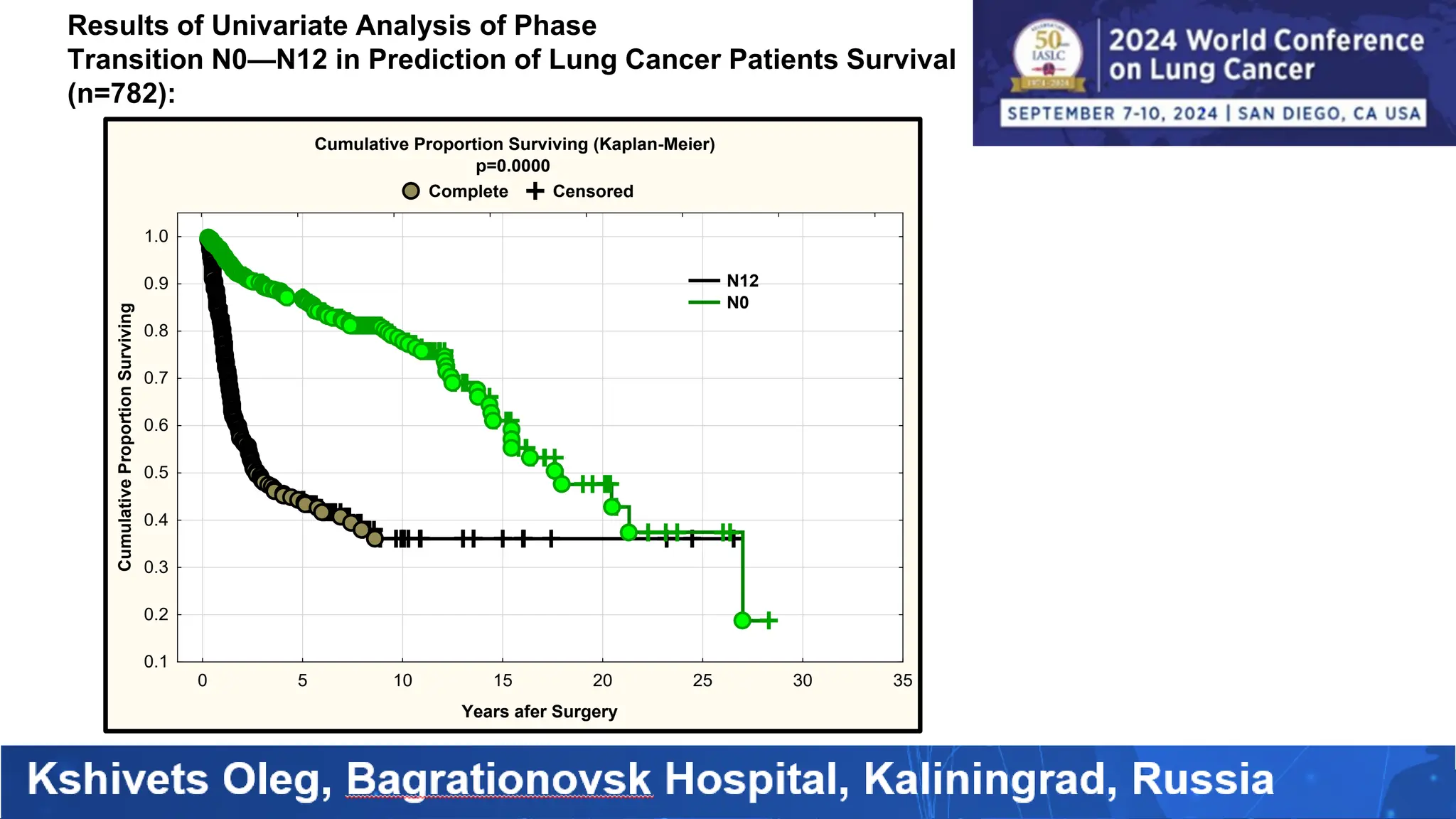
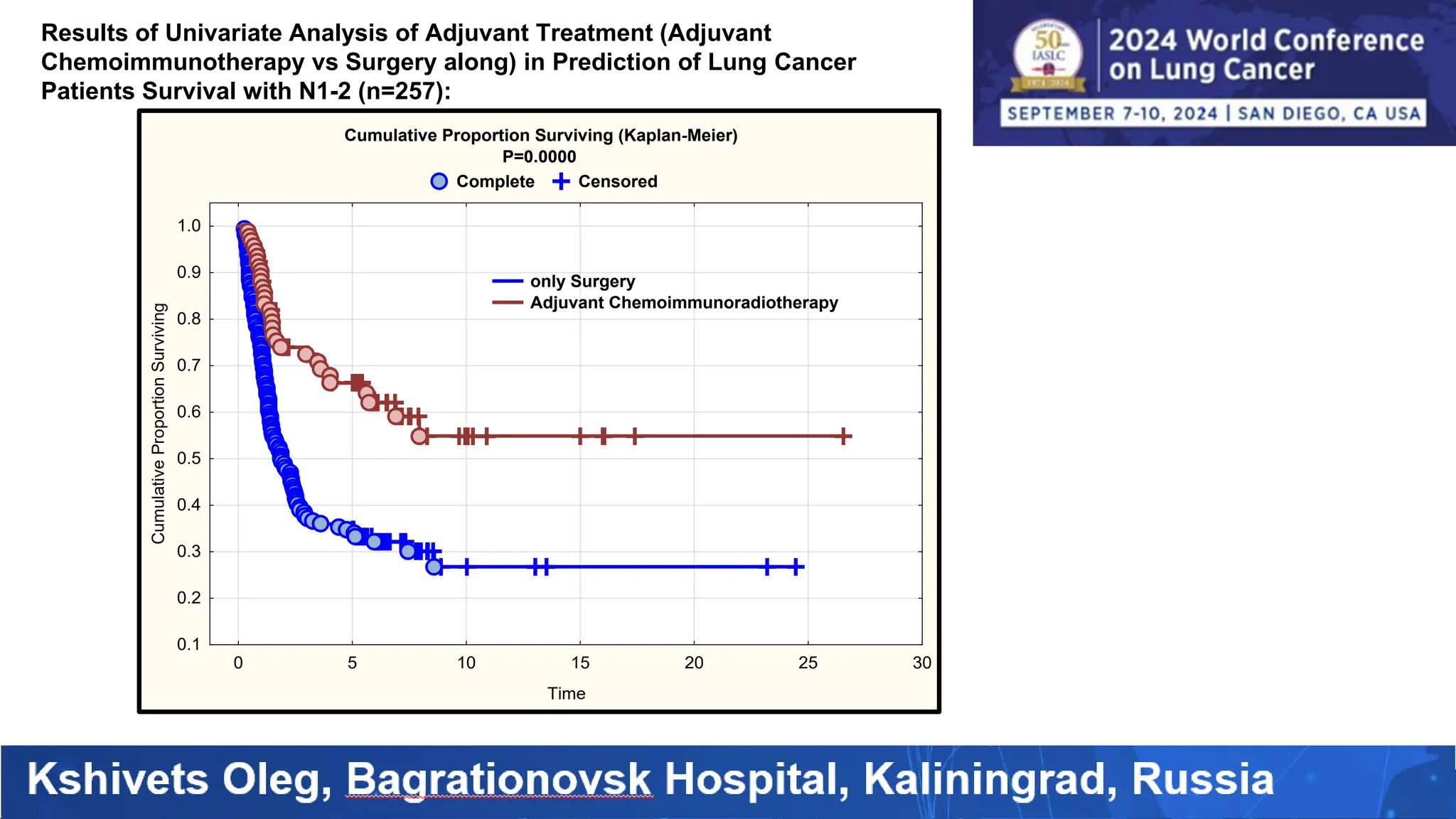
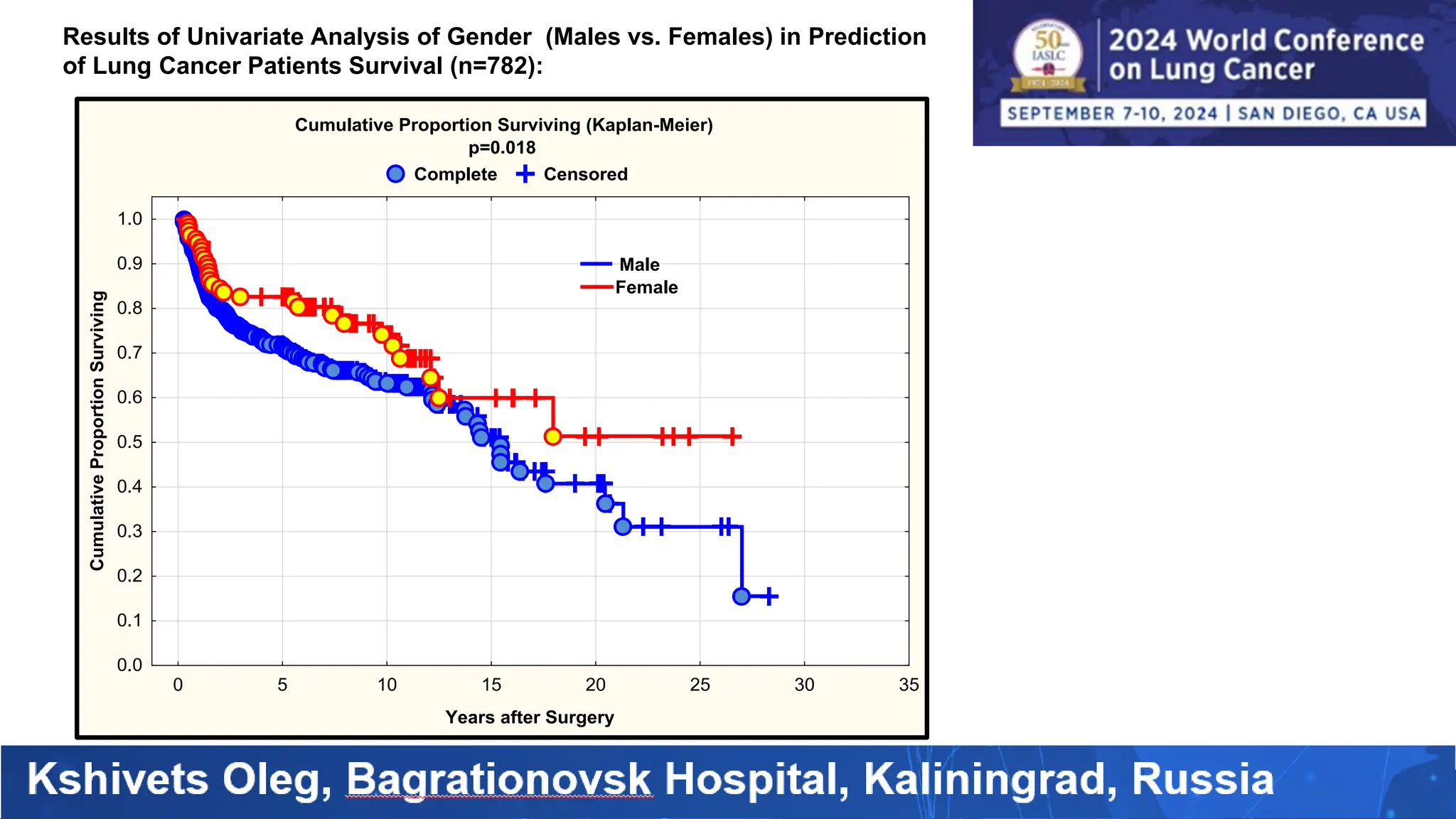
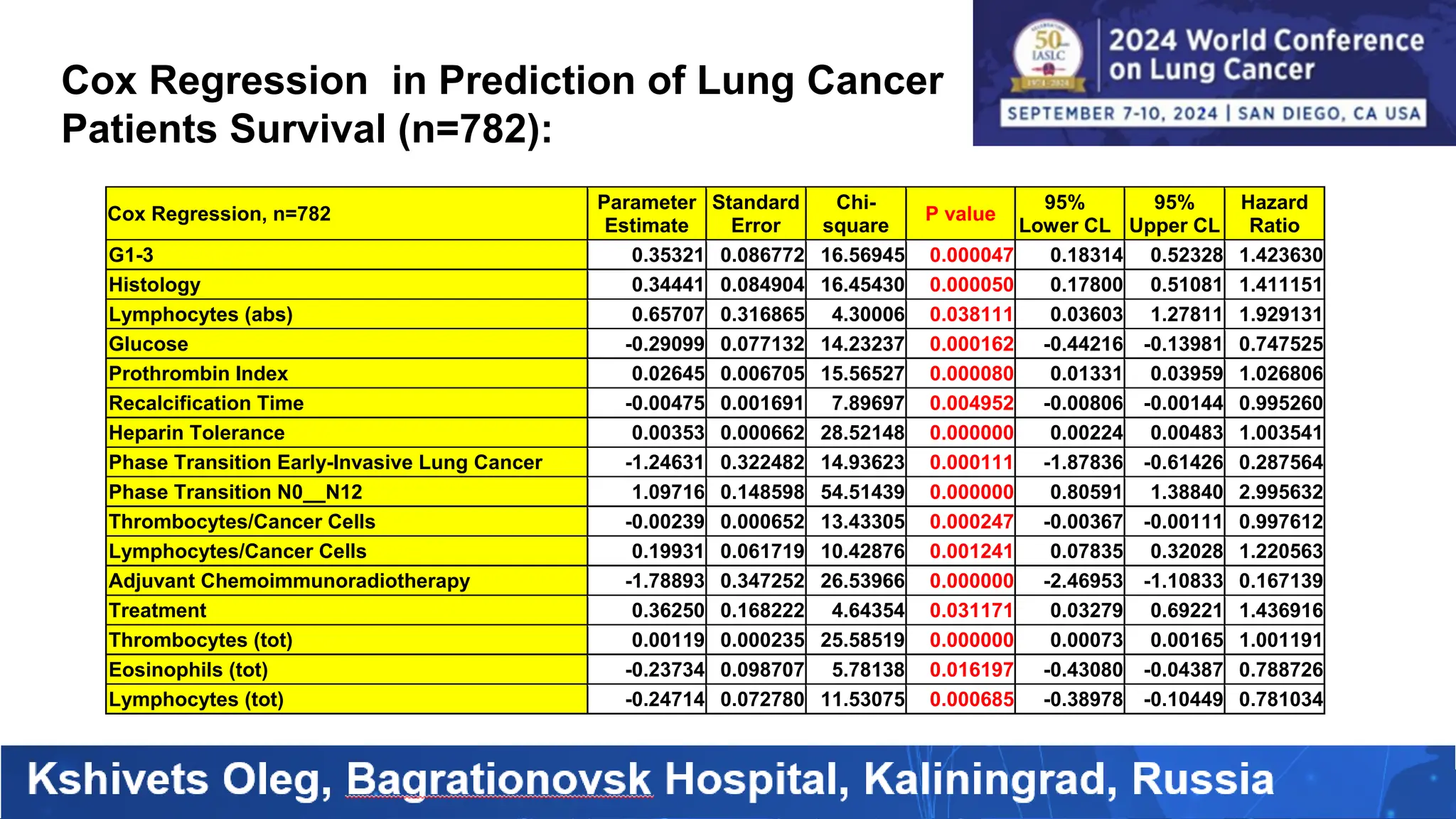
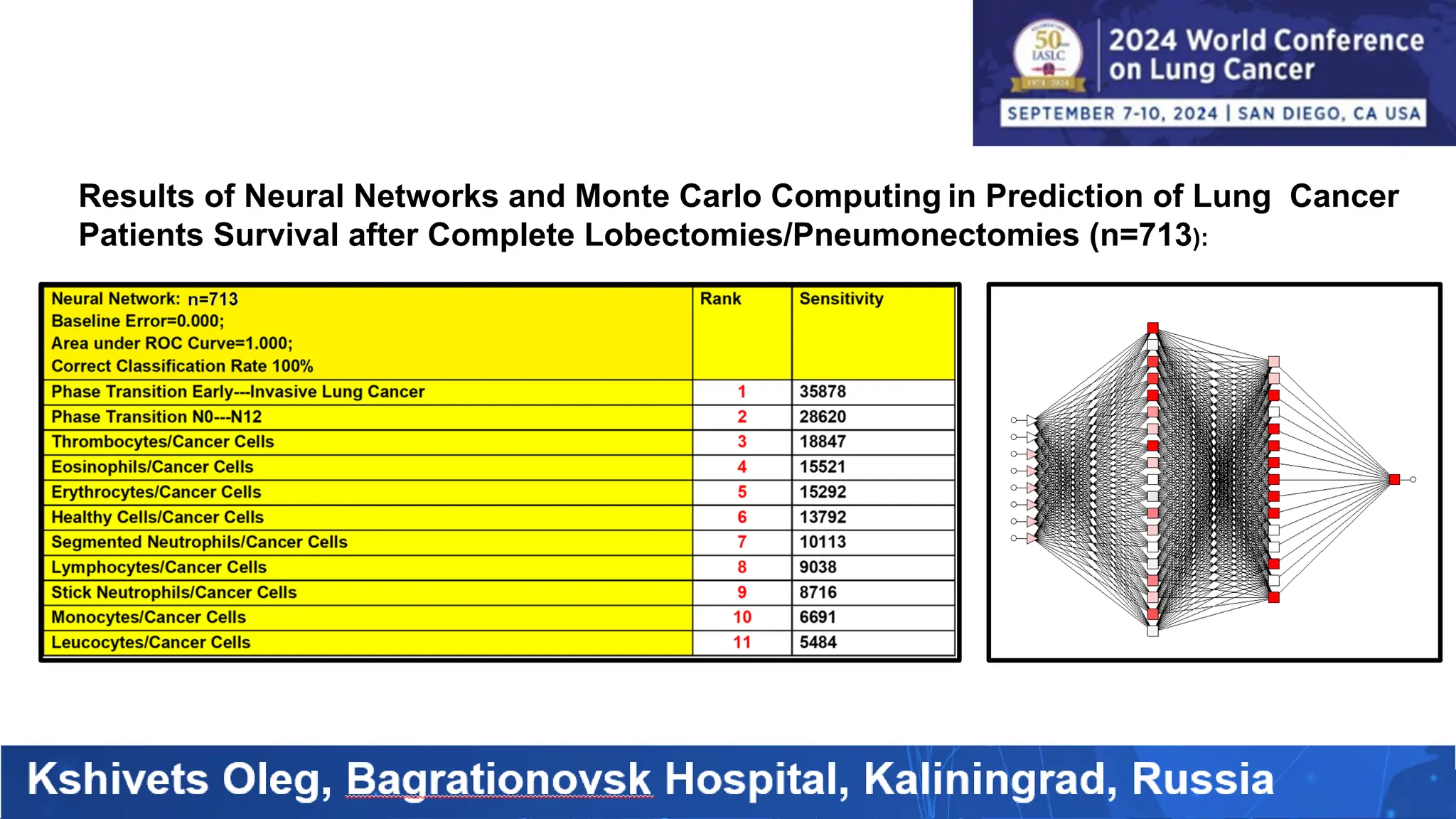
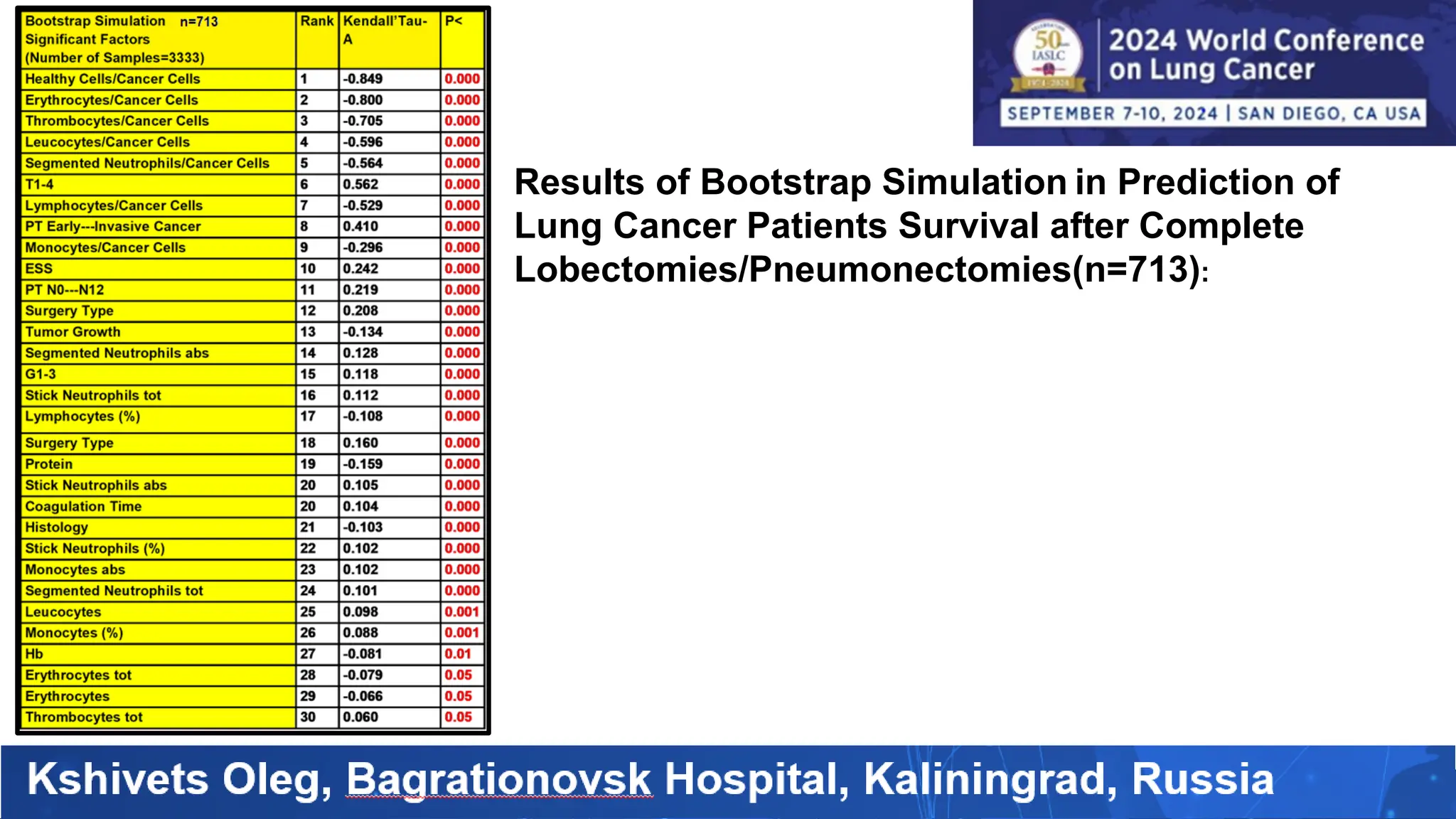
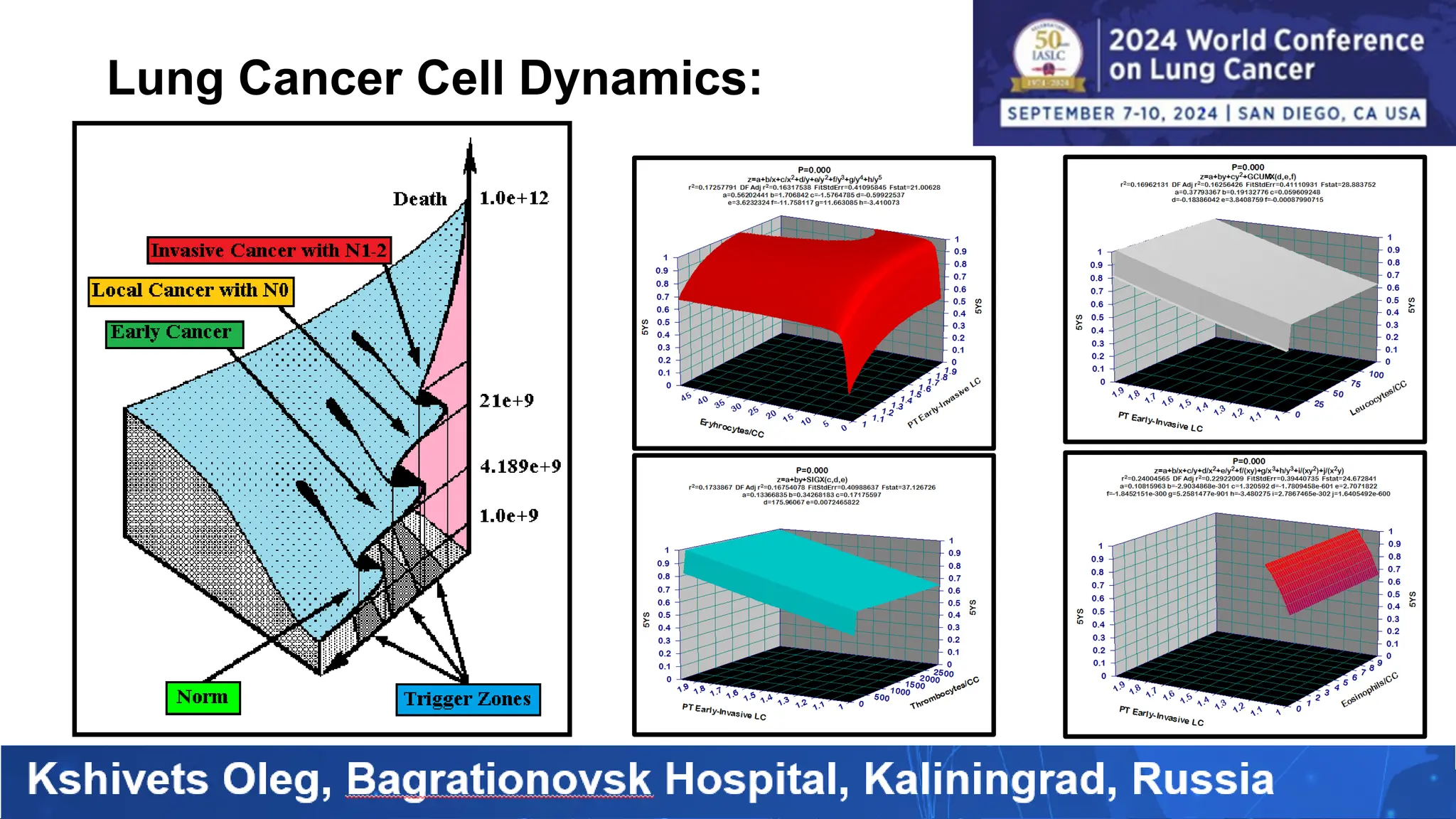
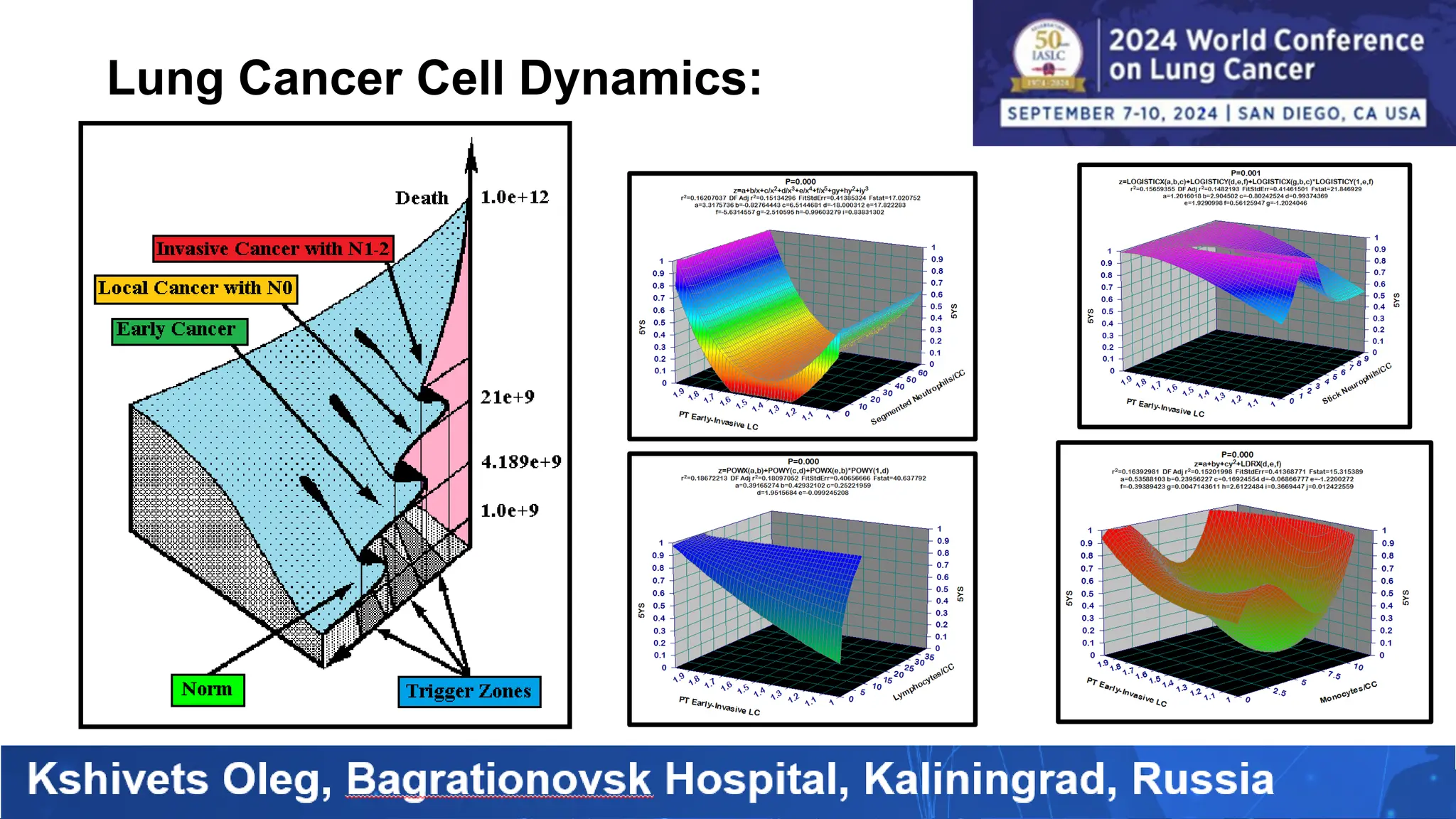
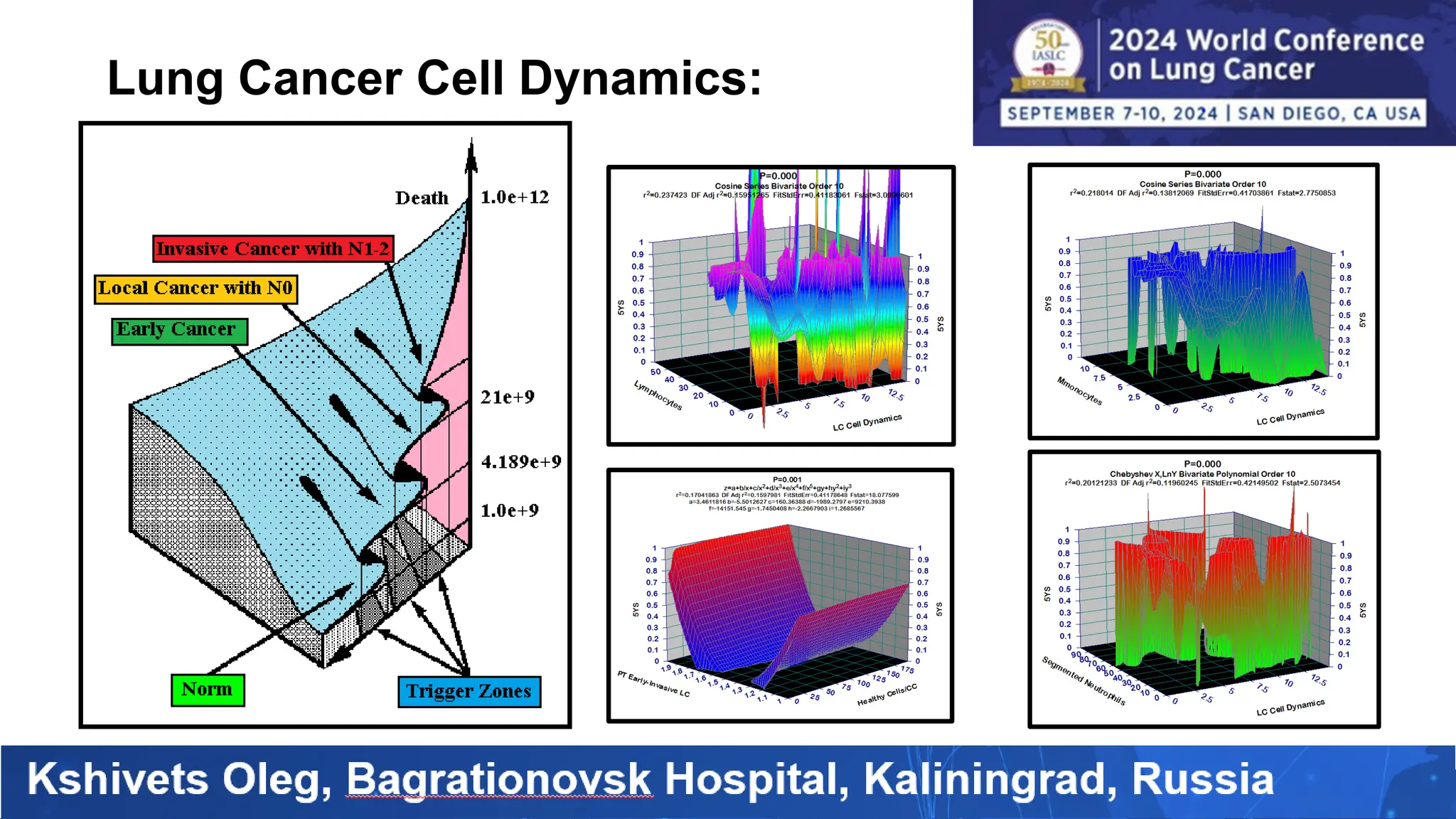
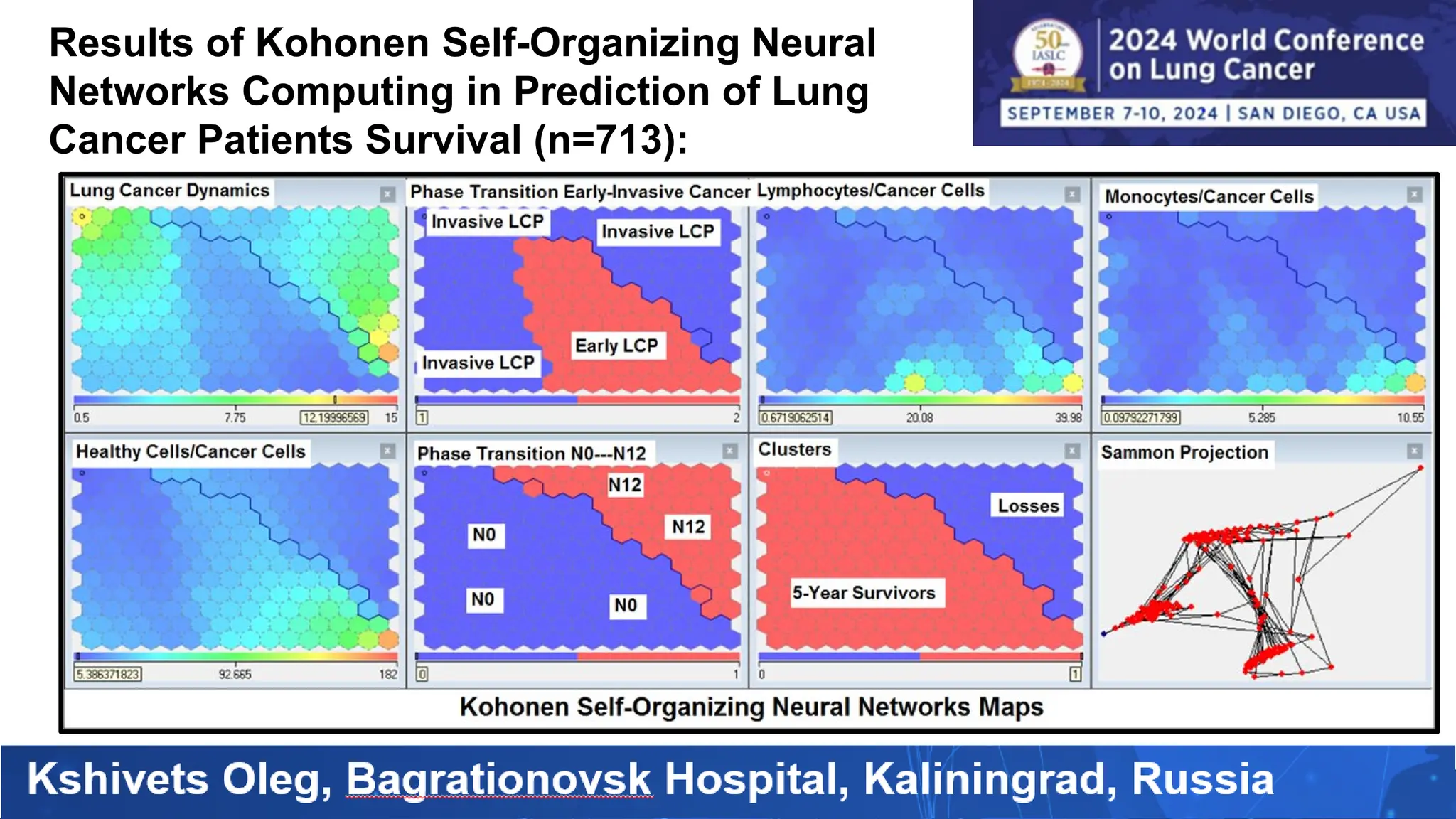
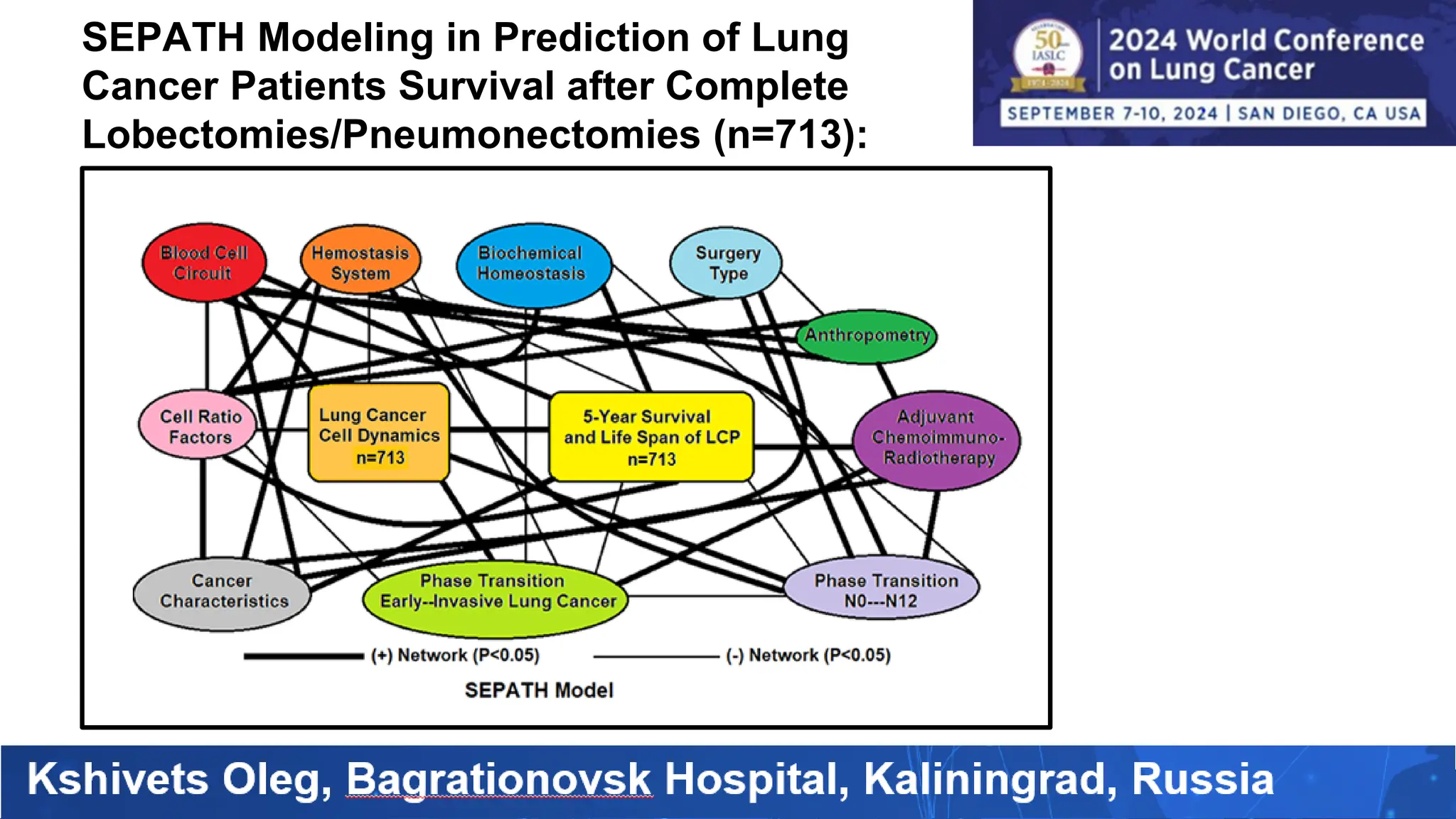
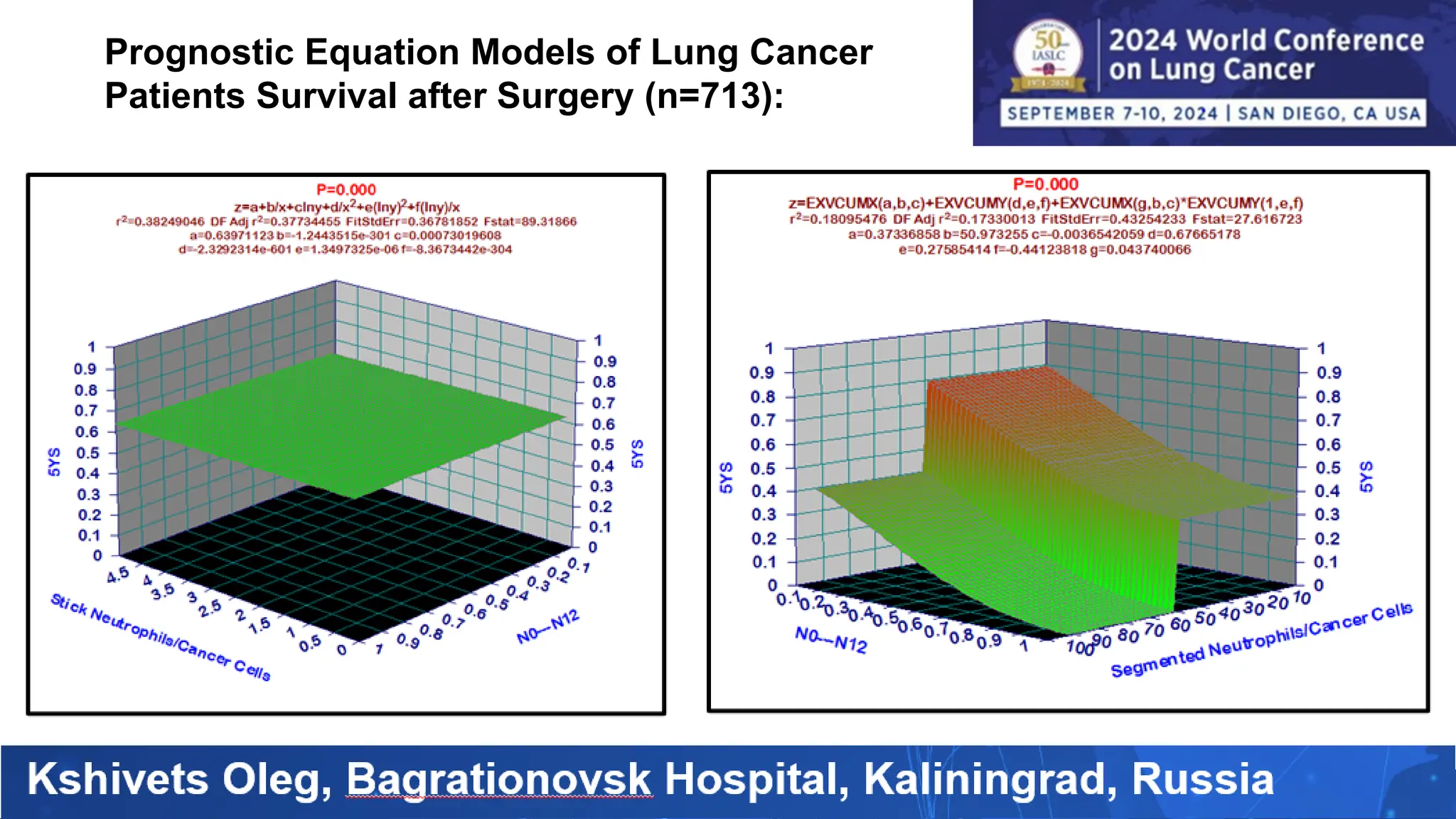
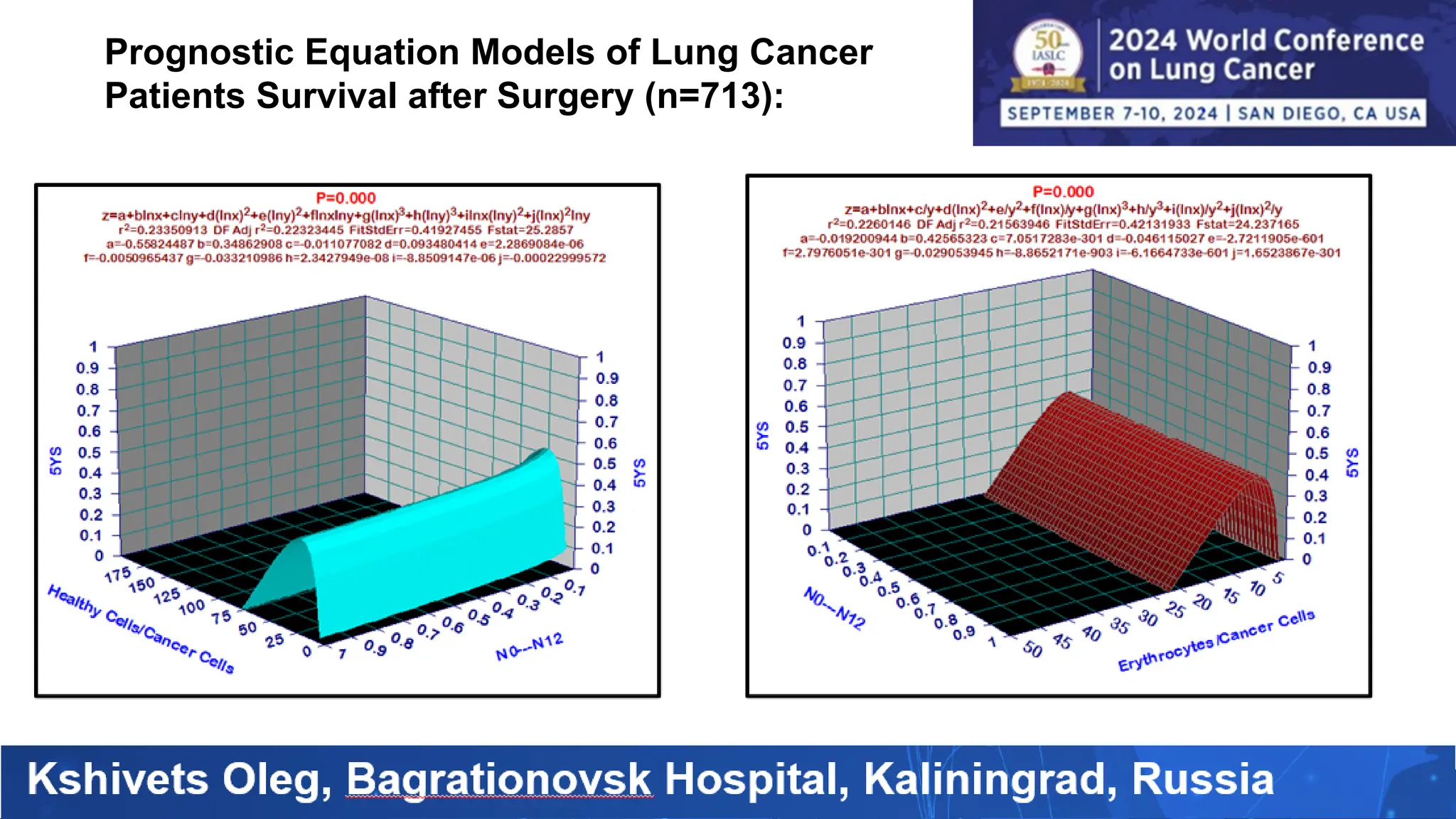
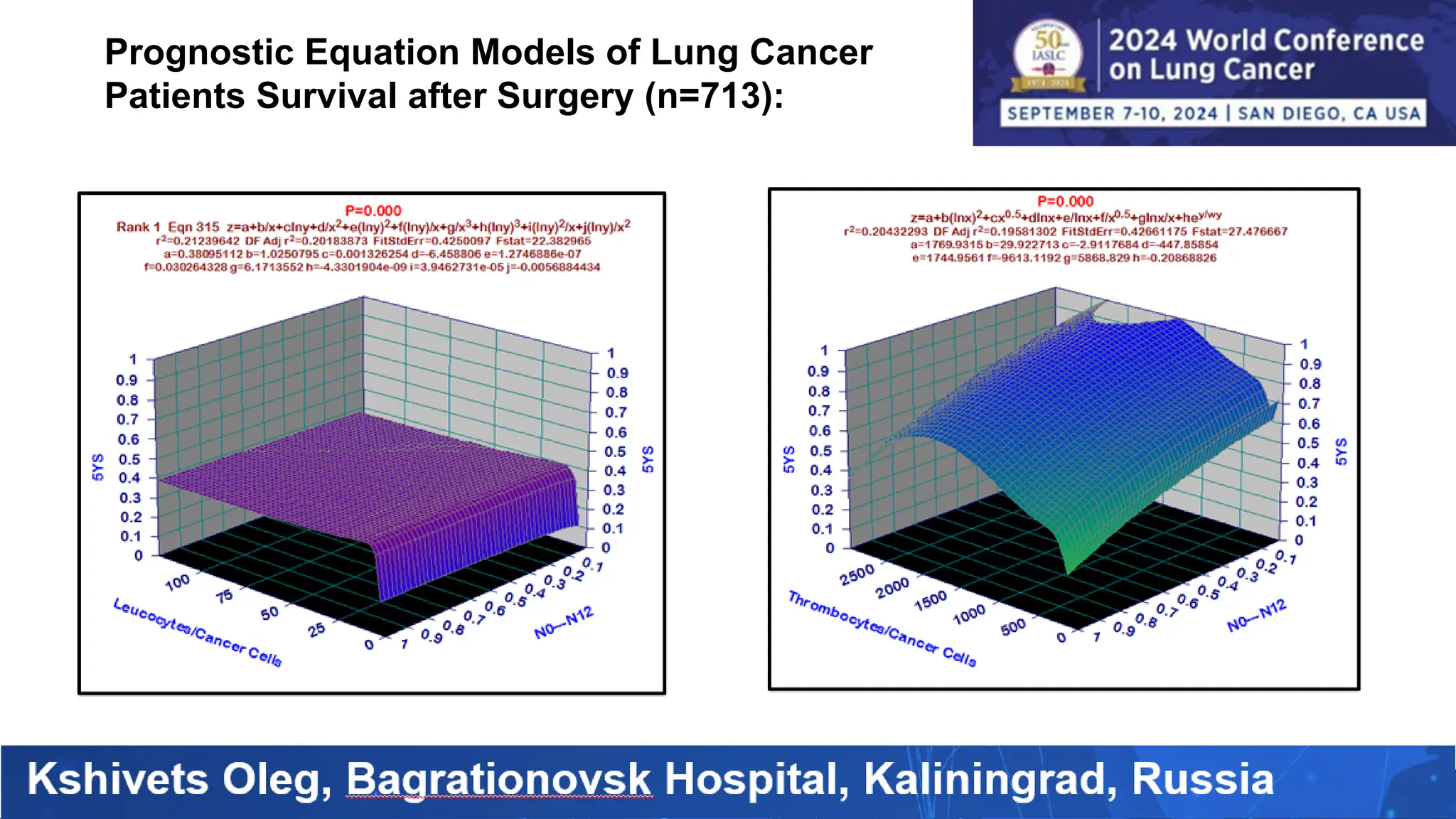
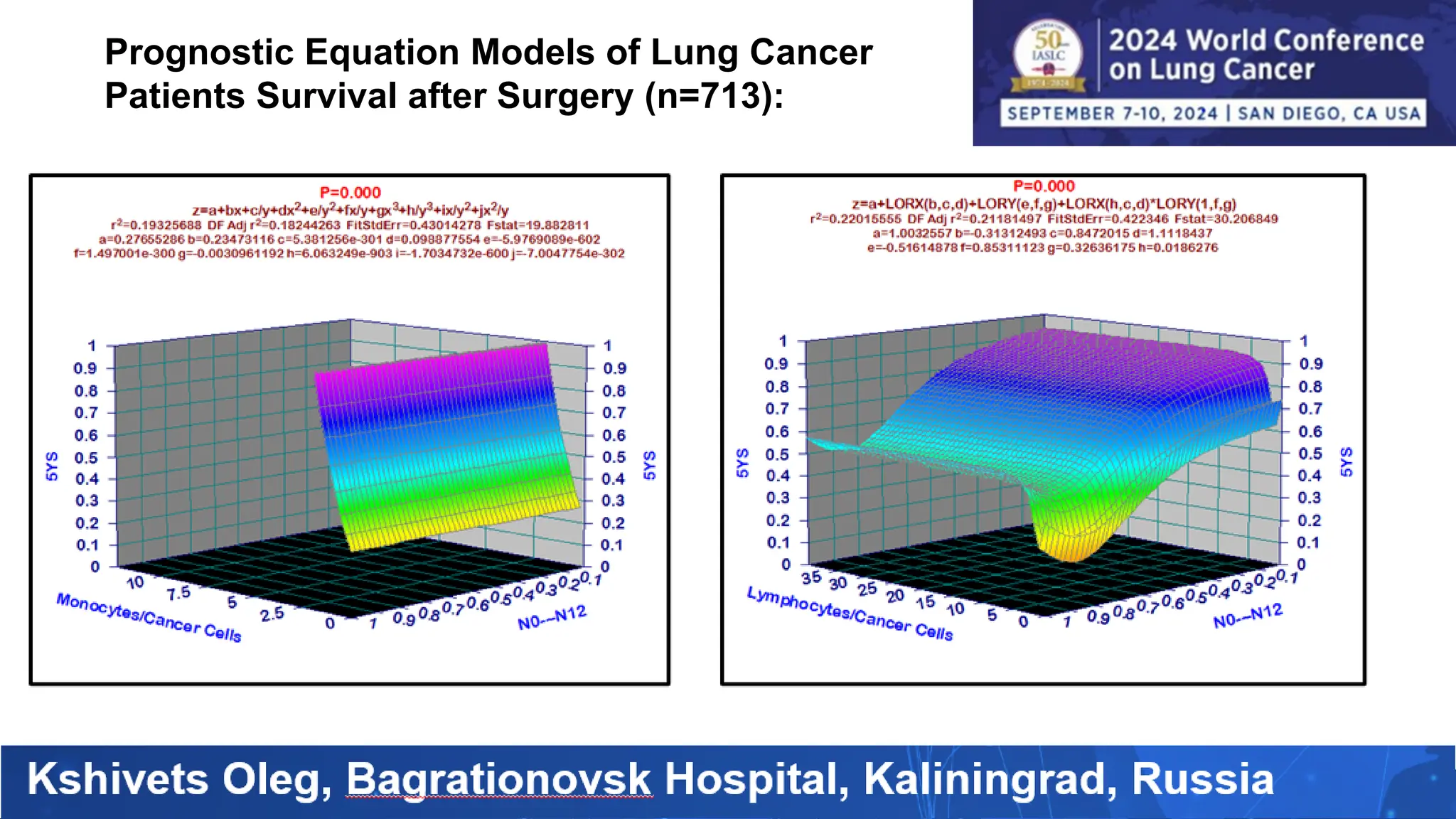
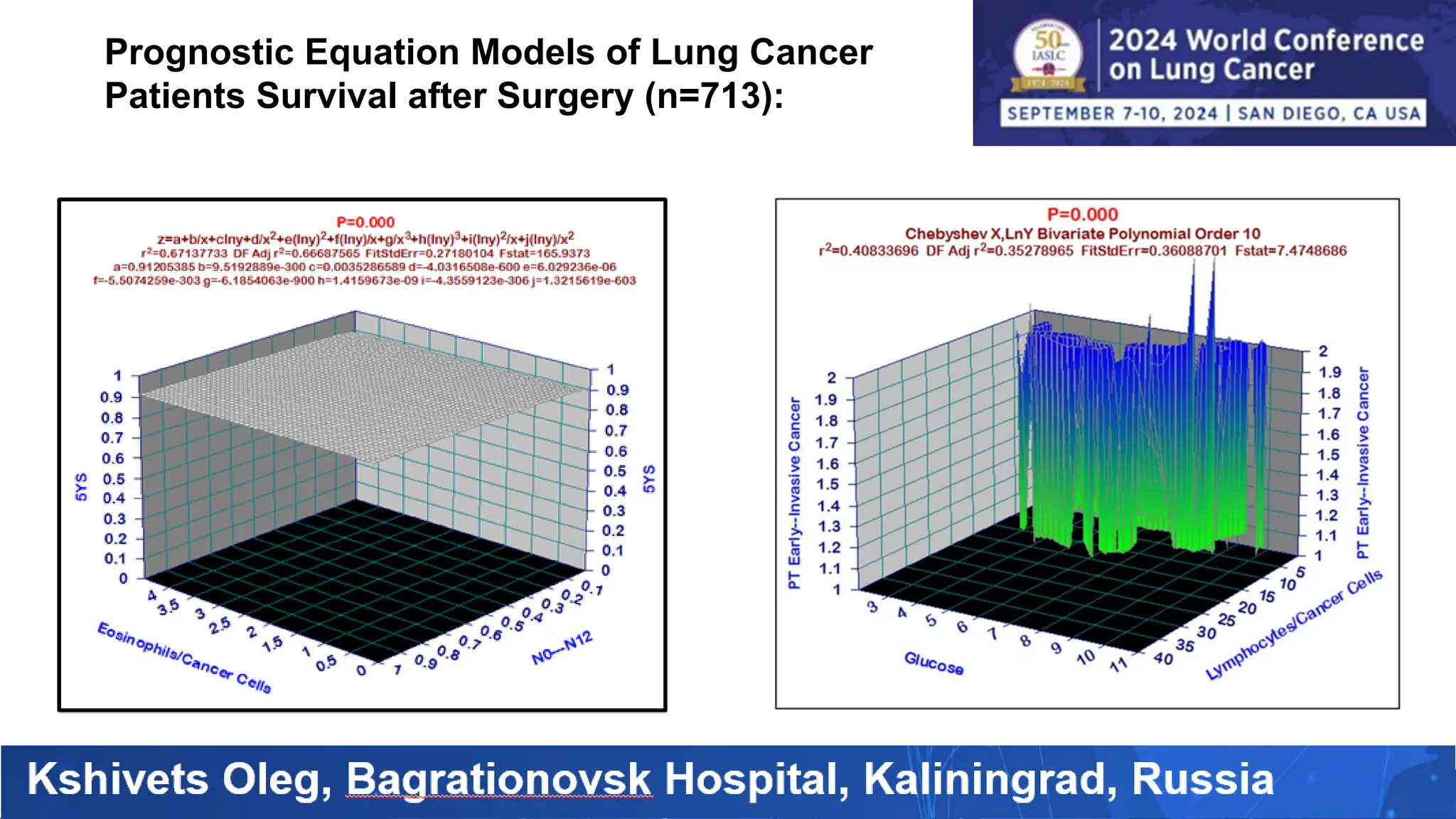
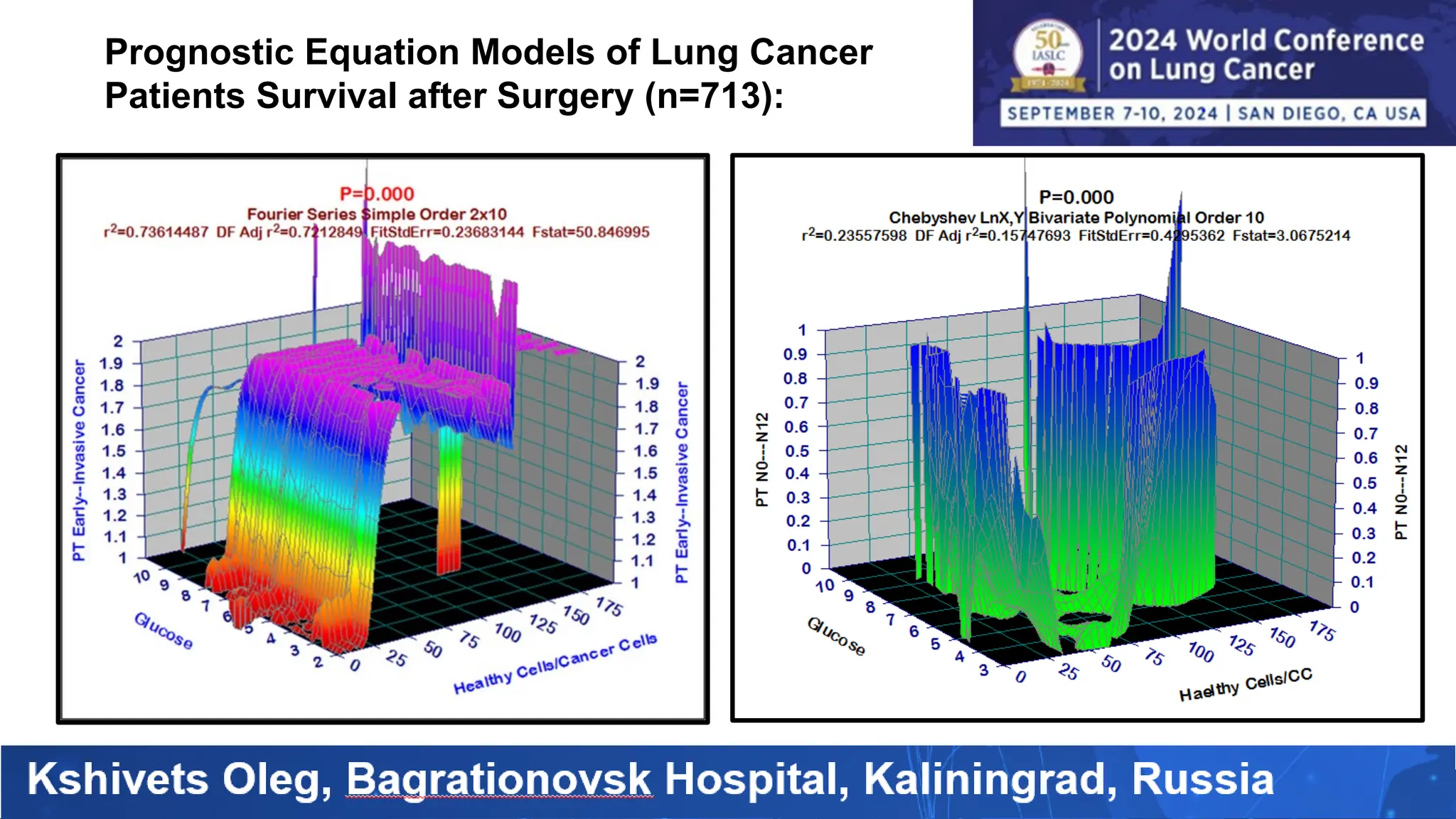
The study analyzed the 5-year survival rates and life spans of 782 non-small cell lung cancer patients who underwent radical surgery, emphasizing the significance of using advanced statistical and artificial intelligence methods for prognostic analysis. Overall, the 5-year survival rate was found to be 73.2%, with various factors including tumor phases, blood cell ratios, and treatment types influencing patient outcomes. The findings advocate for optimal management strategies, including early detection, skilled surgical intervention, and appropriate adjuvant therapies to improve survival rates.