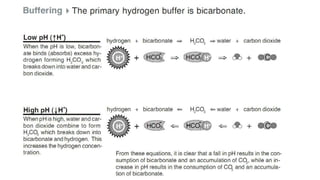
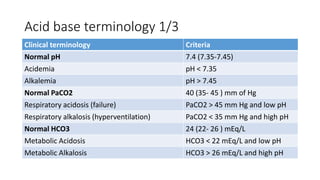
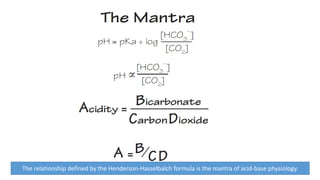
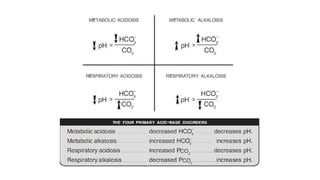
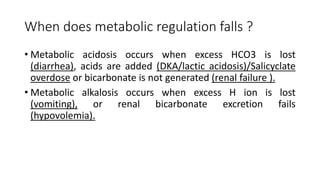
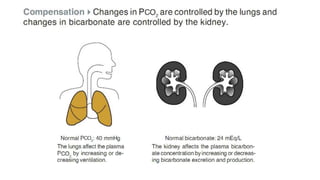
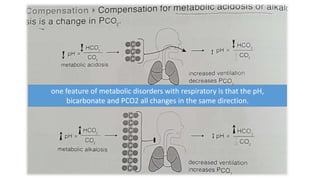
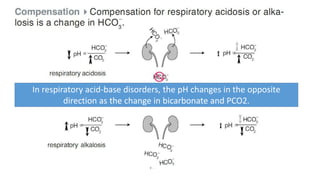
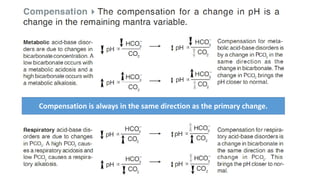
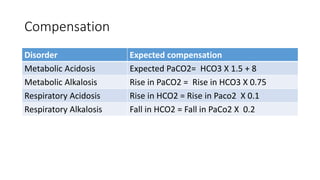
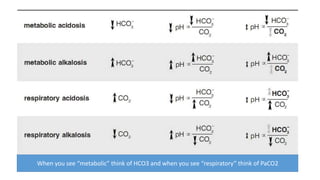
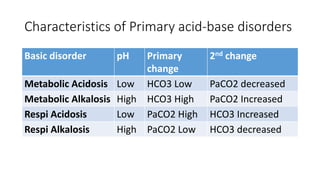
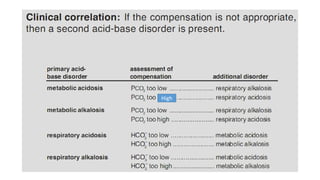
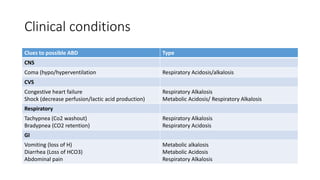
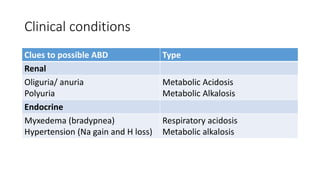
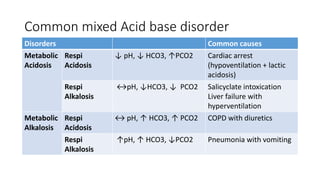
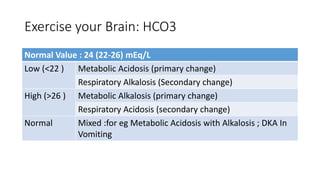
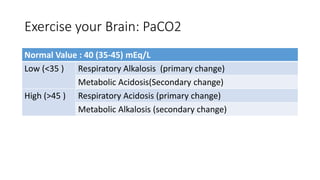
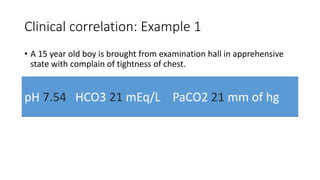
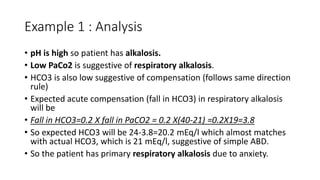
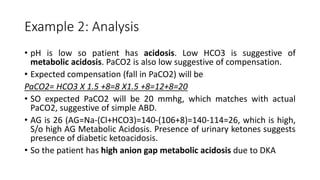
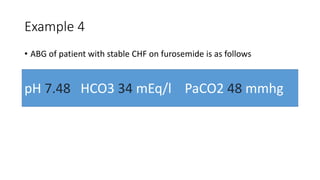
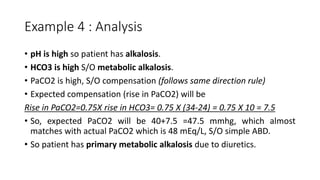
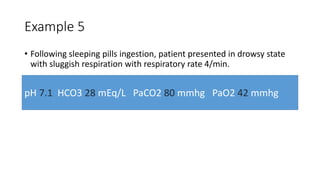
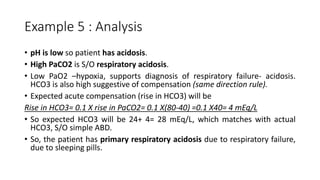
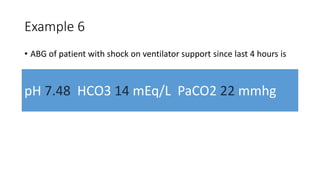
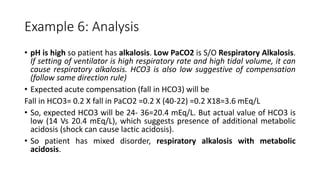
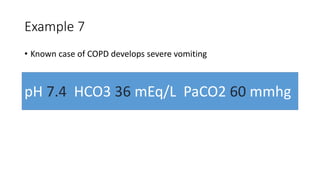
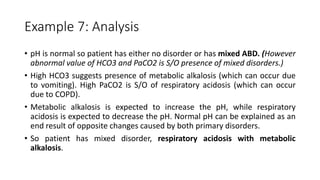
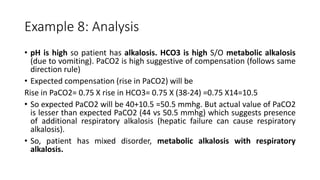
Acid-base disorders occur when pH levels fall outside the normal range of 7.35-7.45. Precise pH regulation is vital for cellular functions and physiological processes. Buffers like bicarbonate help control hydrogen ion concentration. Disorders are classified as metabolic, affecting bicarbonate levels, or respiratory, affecting carbon dioxide levels. The kidneys and lungs work to compensate for changes and return pH to normal ranges through bicarbonate and carbon dioxide regulation. However, compensation cannot fully correct pH without also treating the underlying cause.