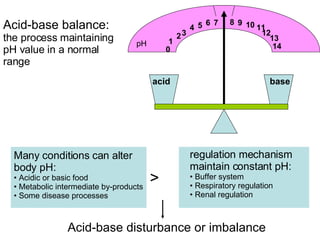
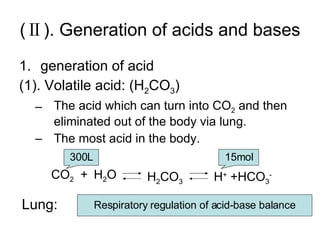
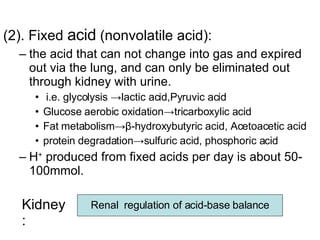
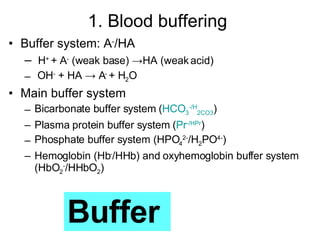
The document discusses acid-base balance and disturbances in the human body. It covers:
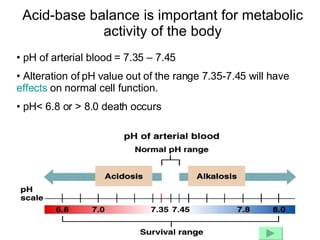
1. The normal pH range of arterial blood and how deviations can affect cell function and lead to death.
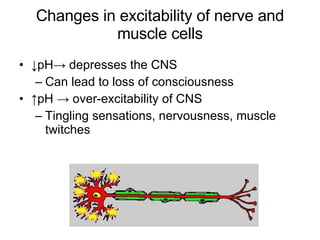
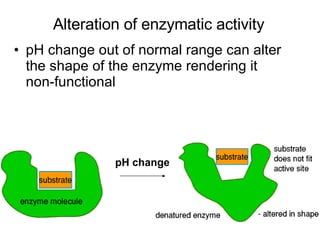
2. How pH changes can alter enzyme activity, nerve and muscle excitability, and potassium levels.
3. The key mechanisms that maintain acid-base balance - buffer systems, respiratory regulation, and renal regulation.




![Normal concentration of H + in body fluid is 4×10 -8 mol/L. pH=- log [H + ] Range of pH is from 0 - 14 Normal pH of blood is 7.35-7.45](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-10-320.jpg)


![Henderson-Hasselbalch equation pH = pK α + lg = pK α + lg = 6.1 + lg = 6.1 + lg = 7.4 dCO 2 = α × PaCO 2 dissolubility pK α = 6.1 α = 0.03 [HCO 3 - ]=24mmol/L PaCO 2 =40mmHg [HCO 3 - ] [H 2 CO 3 ] [HCO 3 - ] α · PaCO 2 24 0.03 × 40 20 1](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-15-320.jpg)
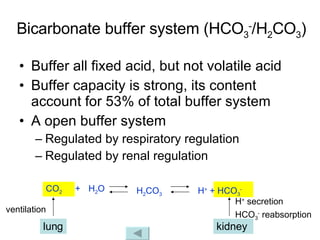

![2. Respiratory regulation The lung regulates the ratio of [HCO 3 - ]/[H 2 CO 3 ] to approach 20/1 by controlling the alveolar ventilation and further elimination of CO 2 , so as to maintain constant pH value. pH = pK α + lg = pK α + lg [HCO 3 - ] [H 2 CO 3 ] [HCO 3 - ] α · PaCO 2](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-20-320.jpg)
![Regulation of alveolar ventilation (V A ) V A is controlled by respiratory center (at medulla oblongata). Respiratory center senses stimulus coming from: Central chemoreceptor ( located at medulla oblongata ) Alteration of [H + ] in Cerebrospinal fluid ↑ [H+] in Cerebrospinal fluid-> respiratory center exciting-> ↑V A Alteration of PaCO 2 PaCO 2 > 60mmHg-> V A increase 10 times PaCO 2 > 80mmHg-> respiratory center inhibited. Peripheral chemoreceptor ( carotid and aortic body ) ↓ PaO 2 or ↑PaCO 2 or ↑[H + ] ↓ PaO 2 < 60mmHg-> respiratory center exciting-> ↑V A ↓ PaO 2 < 30mmHg-> respiratory center inhibited CO 2 narcosis](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-21-320.jpg)
![How does alteration of alveolar ventilation regulate pH value? ↑ [H + ] in Blood-> rapidly buffered by buffer system such as HCO 3 - /H 2 CO 3 -> ↓ [HCO 3 - ] and ↑ [H 2 CO 3 ] -> [HCO 3 - ]/[H 2 CO 3 ] tend to decrease, while ↑[H + ] can stimulate peripheral chemoreceptor ->respiratory center exciting ->↑alveolar ventilation ->↑CO 2 elimination ->↓PaCO 2 -> [HCO 3 - ] / [H 2 CO 3 ] tends to 20/1 -> pH is maintained. ↓ pH = pK α + lg [HCO 3 - ] [H 2 CO 3 ] ↓ ↑](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-22-320.jpg)
![3. Renal regulation The kidney regulates [HCO 3 - ] through changing acid excretion and bicarbonate conservation , so that the ratio of [HCO 3 - ]/[H 2 CO 3 ] approach 20/1 and pH value is constant. pH = pK α + lg = pK α + lg [HCO 3 - ] [H 2 CO 3 ] [HCO 3 - ] α · PaCO 2](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-23-320.jpg)

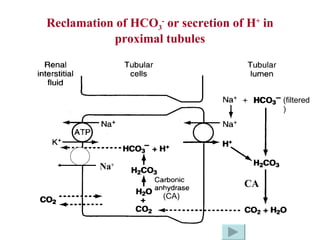
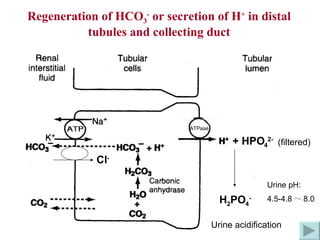

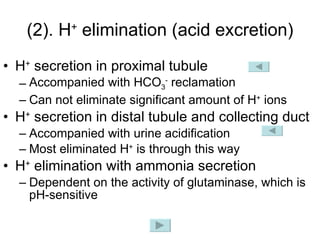
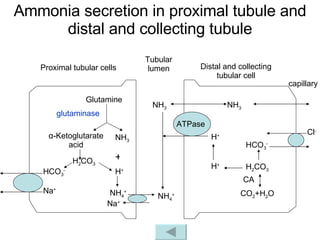
![How does the renal regulation maintain the constant pH value? ↑ [H + ] in Blood-> rapidly buffered by buffer system such as HCO 3 - /H 2 CO 3 -> ↓ [HCO 3 - ] and ↑ [H 2 CO 3 ] -> [HCO 3 - ]/[H 2 CO 3 ] tend to decrease, while ↑[H + ] can stimulate the activity of CA, H + -ATPase and glutaminase-> ↑ secretion of H + and ammonia, ↑reabsorption of HCO 3 - -> [HCO 3 - ] / [H 2 CO 3 ] tends to 20/1 -> pH is maintained. ↑ ↑ pH = pK α + lg [HCO 3 - ] [H 2 CO 3 ] ↓](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-30-320.jpg)
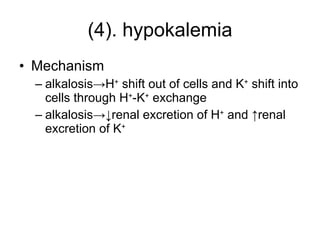
![4. ion exchange between intra- and extracellular compartment & intracellular buffering Intracellular buffer system Phosphate buffer system (HPO 4 2- /H 2 PO 4- ) Hemoglobin (Hb - /HHb) and oxyhemoglobin buffer system (HbO 2 - /HHbO 2 ) Ion exchange between intra- and extracellular compartment i.e. ↑Extracellular [H + ] -> H + shift into cells and K + shift out of cells acidosis-> hyperkalemia alkalosis-> hypokalemia](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-31-320.jpg)
![(Ⅳ). Laboratory tests Essential parameters for acid-base assessment: pH PaCO2 [HCO3-] pH = pK α + lg = pK α + lg [HCO 3 - ] [H 2 CO 3 ] [HCO 3 - ] α · PaCO 2](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-32-320.jpg)
![pH pH=- log [H + ] Normal pH of blood is 7.35-7.45 pH﹤7.35 -> acidosis or acidemia pH﹥7.45 -> alkalosis or alkalemia A normal pH may also represent an abnormal acid-base status](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-33-320.jpg)
![2. PaCO 2 (partial pressure of CO 2 in arterial blood) The pressure formed by dissolved CO 2 in arterial blood. PaCO 2 is equilibrium with H 2 CO 3 PaCO 2 is controlled by respiration hypoventilation->↑ PaCO 2 hyperventilation->↓ PaCO 2 Normal PaCO 2 is 33 ~ 46mmHg, average 40mmHg. pH = pK α + lg = pK α + lg — Respiratory parameter [CO 2 ] dissolved +H 2 O H 2 CO 3 [HCO 3 - ] [H 2 CO 3 ] [HCO 3 - ] α · PaCO 2](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-34-320.jpg)
![3. [HCO 3 - ] HCO 3 - is the most abundant buffer base (53%) [HCO 3 - ] reflects the acid-base load of the body, which is controlled by metabolic state. i.e. ↑ H + load-> HCO 3 - content will decrease for neutralizing H + ↑ OH - load-> HCO 3 - content will increase because combination of H 2 CO 3 with OH - leads to increased formation of HCO 3 - [HCO 3 - ] also reflects the function of renal tubule(HCO 3 - reclamation and regeneration), while the renal reabsorption of HCO 3 - is controlled by pH status Normal arterial blood [HCO 3 - ] is 22-27mmol/L, average 24mmol/L — metabolic parameter](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-35-320.jpg)
![4. Anion gap (AG) AG= unmeasured anions – unmeasured cations AG=[Na + ] - ([Cl - ] + [HCO 3 - ]) Normal AG is 12±2 mmol/L ↑ AG ↑ unmeasured anions Including phosphates, sulfates, organic acids, and protein anions Suggest an increased acumulation of metabolic acids in the plasma and metabolic acidosis ↓ AG ↓ unmeasured anions Albumin decrease ↑ unmeasured cations Hyperkalemia, hypercalcemia, and so on Na + Cl - HCO 3 - UA UC Measured cation Measured anion](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-36-320.jpg)

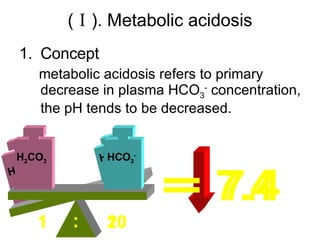
![If [HCO 3 - ] primarily ↓, pH tends to be ↓— metabolic acidosis If [HCO 3 - ] primarily ↑, pH tends to be ↑— metabolic alkalosis If PaCO 2 primarily ↑, pH tends to be ↓— respiratory acidosis If PaCO 2 primarily ↓, pH tends to be ↑— respiratory alkalosis Primary change Secondary change Classification of simple acid-base disorders [HCO 3 - ] Primarily ↓, from 20 to 10 If [H 2 CO 3 ] secondarily↓, from 1 to 0.5-> pH normal If [H 2 CO 3 ] secondarily ↓, from 1 to 0.8-> pH↓ Metabolic parameter pH = pK α + lg [HCO 3 - ] [H 2 CO 3 ] = 6.1 + lg [HCO 3 - ] α · PaCO 2 = 6.1+ lg 20 1 =7.4 respiratory parameter](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-38-320.jpg)
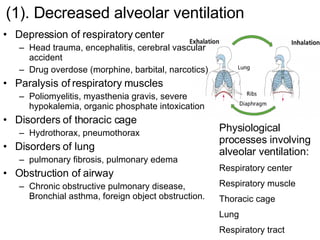
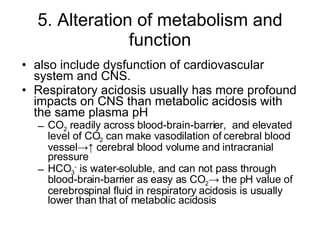
![2. Primary causes Central change: ↓ [HCO 3 - ] direct excessive loss of HCO 3 - indirect loss of HCO 3 - for buffering increased nonvolatile acid Excessive intake of nonvolatile acid Excessive production of nonvolatile acid Decreased renal excretion of acid](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-40-320.jpg)

![Respiratory regulation ↑ [H + ] -> stimulate peripheral chemoreceptor in carotid and aortic body -> respiratory center excitation -> hyperpnea ->↑CO 2 elimination and ↓PaCO 2 -> [HCO 3 - ] /[H 2 CO 3 ] near 20/1 -> pH is maintained i.e. pH 7.4->7.0, alveolar ventilation 4L/min->30L/min](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-47-320.jpg)
![Intracellular buffering ↑ [H + ] in ECF-> H + move in cells through H + -K + exchange and K + move out of cells-> hyperkalemia is resulted in](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-48-320.jpg)
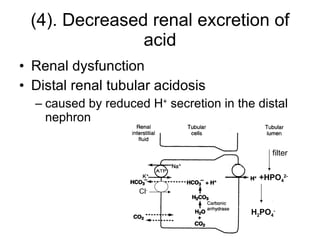
![Renal regulation ↑ [H + ] in ECF-> ↑activity of carbonic anhydrase, H + -ATPase and glutaminase ↑ Renal tubular secretion of H + ↑ Renal tubular reaborption of HCO 3 - ↑ Renal tubular secretion of ammonia (3-5days) ↑ [HCO 3 - ], and [HCO 3 - ] /[H 2 CO 3 ] near 20/1 pH is near to normal](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-49-320.jpg)

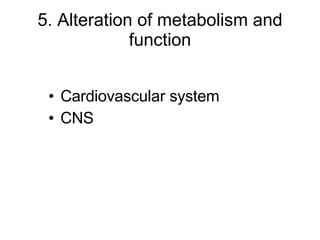
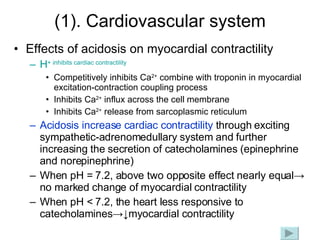
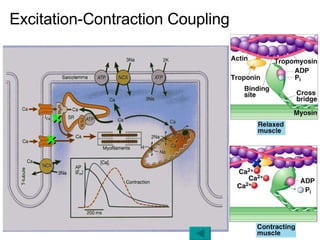
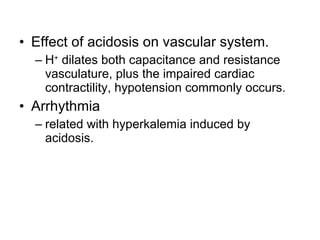
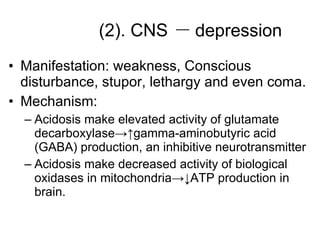
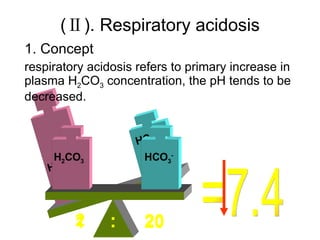


![Intracellular buffering Main compensatory method of acute respiratory acidosis [HCO 3 - ] increase 1mmol/L per increased 10mmHg of PaCO 2 Acute respiratory acidosis is usually decompensated Carbonic anhydrase When PaCO 2 is 60mmHg, then PaCO 2 increase 20mmHg, and through compensation [HCO 3 - ] increase 2mmol/L, pH=6.1+lg(24+2)/0.03×60=6.1+lg26/1.8 ↑ [H 2 CO 3 ] H 2 CO 3 HCO 3 - H 2 O CO 2 Cl - H + H + CO 2 H 2 O H + HCO 3 - + + + + K + HCO 3 - Buffered by intracellular buffer system ICF ECF](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-62-320.jpg)
![Renal regulation Main compensatory method of chronic respiratory acidosis Detailed process Increased PaCO 2 and [H + ] can elevate the activity of carbonic anhydrase and glutaminase in renal tubular cells->renal tubular secretion of H + and ammonia increase and renal tubular reabsorption of HCO 3 - increase, which can compensate the relative deficit of HCO 3 - Through renal compensation, [HCO 3 - ] can increase 3.5-4.5mmol/L per increased 10mmHg of PaCO 2 . Chronic respiratory acidosis is usually compensated](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-63-320.jpg)

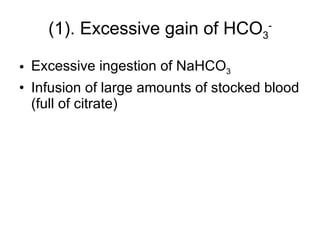
![Central change: ↑ [HCO 3 - ] excessive gain of HCO 3 - excessive loss of H + Volume contraction 2. Primary causes](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-67-320.jpg)

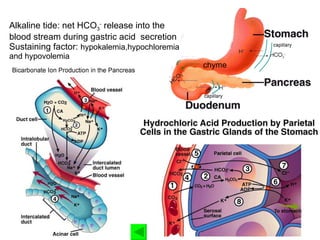
![Aldosterone promote renal excretion of H + ADS promote H + secretion through H + –ATPase in collecting duct->↑HCO 3 - reabsorption ADS promote sodium retention and potassium excretion->↓ [K + ] in ECF->↑ K + shift from ICF to ECF and ↑ H + shift from ECF to ICF through H + -K + exchange ->↑ [H + ] in ICF -> renal excretion of H + increase ->↑HCO 3 - reabsorption](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-71-320.jpg)
![Diuretic promote renal excretion of H + Diuretic inhibit the reabsorption of Na + and Cl - in henle’s loop and early distal tublule->↑ [Na + ], [Cl - ] in distal tubule-> promote secretion of H + and K + in distal tubule and collecting duct in order to increase Na + reabsorption->↑HCO 3 - reabsorption diuretic->↓ECF volume->↑ADS secretion](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-72-320.jpg)

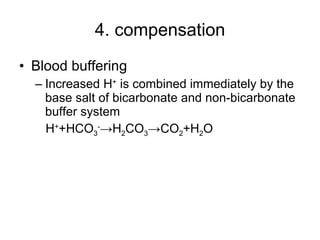
![3. compensation Blood buffering During metabolic alkalosis, ↓[H + ] ECF and ↑ [OH - ] ECF -> OH - can be buffered by weak acids, such as H 2 CO 3 ->↑ [HCO 3 - ] Ion exchange between intra- and extra-cell In alkalosis, ↓[H + ] ECF ->through H + - K + exchange, H + shift out of cells and K + shift into cells->hypokalemia](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-74-320.jpg)
![Respiratory regulation ↓ [H + ] ->inhibition of respiratory center ->↓alveolar ventilation-> ↑PaCO 2 or [H 2 CO 3 ] -> [HCO 3 - ]/ [H 2 CO 3 ] approach 20/1 Respiratory regulation is limited and seldom make complete compensation ↓ Alveolar ventilation -> ↑ PaCO 2 ->but when PaCO 2 >60mmHg, respiratory center is excited->respiration deepen and quicken->↑CO2 expiration so compensatory limit of secondary increase of PaCO 2 is 55mmHg](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-75-320.jpg)
![Renal regulation ↓ [H + ] -> ↓the activity of carbonic anhydrase and glutaminase in renal tubular cell -> ↓ renal secretion of H + and ammonia, ↓renal reabsorption of HCO3 - ->↓ [HCO3 - ] in plasma-> [HCO 3 - ]/ [H 2 CO 3 ] approach 20/1 The increased renal excretion of HCO3 - peaks at 3-5 days, so this regulation is not useful for acute metabolic alkalosis.](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-76-320.jpg)
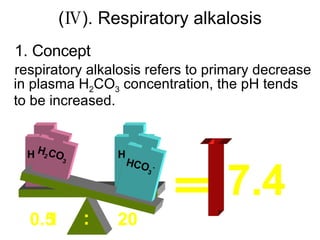

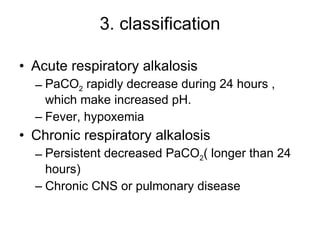

![ion exchange between intra- and extra-cells and intracellular buffering Main compensatory method of acute respiratory alkalosis Through above compensation, [HCO 3 - ] decrease 2mmol/L per decreased 10mmHg of PaCO 2 Acute respiratory alkalosis is usually decompensated H 2 CO 3 H 2 CO 3 [HCO 3 - ] relatively increase H 2 O CO 2 Cl - H + CO 2 H 2 O H + HCO 3 - + + + + K + HCO 3 - ICF ECF H 2 CO 3 HCO 3 - H + primary decrease of [H 2 CO 3 ] When PaCO2 is 20mmHg, then PaCO2 decrease 20mmHg, and through compensation [HCO3-] decrease 4mmol/L, pH=6.1+lg(24-4)/0.03×20=6.1+lg20/0.6=7.63](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-87-320.jpg)
![Renal regulation Main compensatory method of chronic respiratory alkalosis Detailed process Decreased PaCO 2 and [H + ] can decrease the activity of carbonic anhydrase and glutaminase in renal tubular cells->renal tubular secretion of H + and ammonia decrease and renal tubular reabsorption of HCO 3 - decrease->↑renal excretion of HCO 3 - In chronic respiratory alkalosis, through renal regulation and intracellular buffering, [HCO 3 - ] can decrease 5mmol/L per decreased 10mmHg of PaCO 2 . Chronic respiratory alkalosis is usually compensated](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-88-320.jpg)
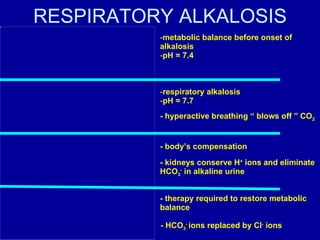

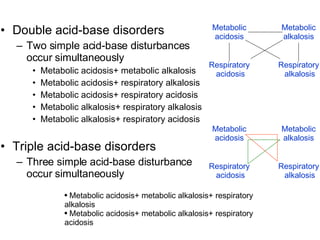
![1. Metabolic acidosis+ Metabolic alkalosis Causes Diarrhea and vomiting Lactic acidosis and vomiting Ketoacidosis ang hypokalemia Characteristics [HCO 3 - ]: ↑/normal/↓ pH: ↑/normal/↓](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-93-320.jpg)
![2. Metabolic acidosis+ Respiratory alkalosis Causes: Salicylate toxication Diabetes mellitus, renal failure or cardiopulmonary diseases companied with fever Chronic liver disease(with increased blood ammonia) companied with renal failure Characteristics: [HCO 3 - ]: ↓ PaCO 2 : ↓ pH: ↑/normal/↓](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-94-320.jpg)
![3. Metabolic acidosis+ Respiratory acidosis Causes: Cardiopulmonary resuscitation Pulmonary edema Chronic obstructive pulmonary disease Characteristics: [HCO 3 - ]: ↓ PaCO 2 : ↑ pH: ↓](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-95-320.jpg)
![4. Metabolic alkalosis+ Respiratory alkalosis Causes: Fever accompanied with vomiting Hepatic failure(with increased blood ammonia) accompanied with inappropriate use of diuretic Characteristics: [HCO 3 - ]: ↑ PaCO 2 : ↓ pH: ↑](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-96-320.jpg)
![5. Metabolic alkalosis+ Respiratory acidosis Causes: Chronic obstructive pulmonary disease companied with use of diuretics or glucocorticoid Characteristics: [HCO 3 - ]: ↑ PaCO 2 : ↑ pH: ↑/normal/↓](https://image.slidesharecdn.com/acidbase-balance-and-disturbance-1208278803749030-8/85/Acid-Base-Balance-And-Disturbance-97-320.jpg)