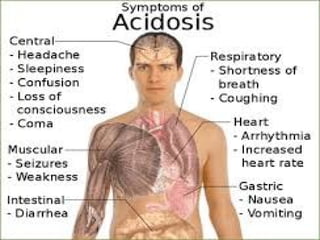
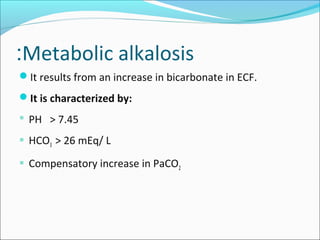
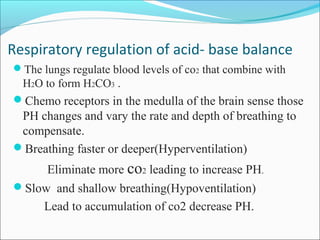
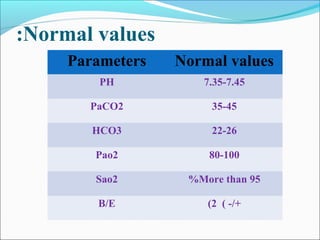
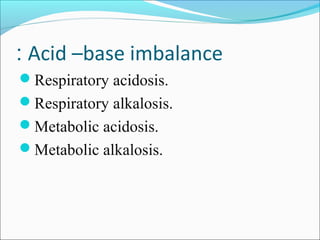
This document discusses acid-base balance and imbalance. It defines key terms like pH, acids, and bases. The body regulates acid-base balance through buffering systems, respiratory compensation, and renal compensation. Acid-base imbalance can be diagnosed using arterial blood gases and anion gap tests. The main types of imbalance are respiratory acidosis and alkalosis from lung issues, and metabolic acidosis and alkalosis from kidney or production problems. Causes, signs, and compensation methods are described for each type.













![ABG parameters:
pH [H+
]
PCO2 Partial pressure CO2
PO2 Partial pressure O2
HCO3 Bicarbonate
BE Base excess
SaO2 Oxygen Saturation](https://image.slidesharecdn.com/acid-baseimbalances2018-180112200052/85/Acid-base-imbalances-2018-17-320.jpg)



![Anion Gap in Plasma
The difference between measured major positive and negative
charges.
[Na+
] -[Cl-
] - [HCO3-
]
The normal value is (8-14) mEq/L
The sum of measured cations is grater than of measured anions
due to the presence of unmeasured anions (proteins
,phosphates, sulfates, and organic acids as lactic acids, and
ketones acids).
An increased anion gap may be the only clue that metabolic
acidosis is present in a mixed acid-base disorder.](https://image.slidesharecdn.com/acid-baseimbalances2018-180112200052/85/Acid-base-imbalances-2018-22-320.jpg)





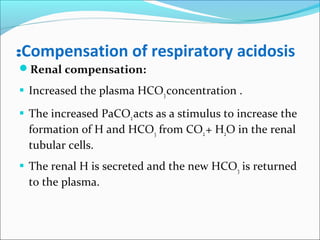
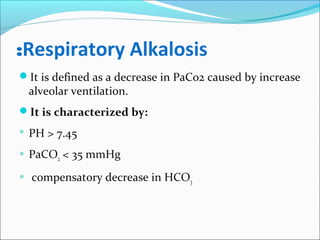


![Compensation of respiratory alkalosis
Renal compensation:
The kidneys decrease plasma [ HCO3 ]:
Decrease reabsorption of the filtered HCO3.
The decreased CO2 decreases the generation of H by the
tubular epithelial cells.](https://image.slidesharecdn.com/acid-baseimbalances2018-180112200052/85/Acid-base-imbalances-2018-34-320.jpg)