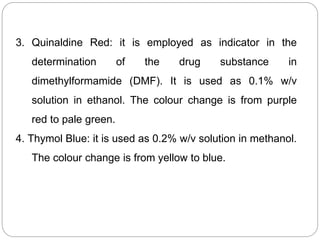
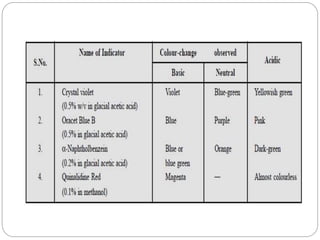
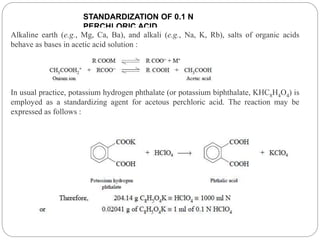
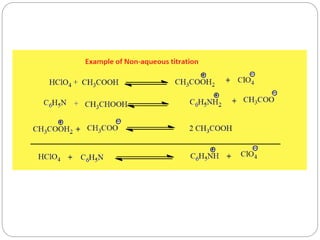
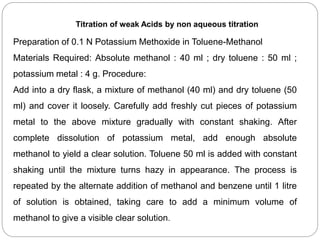
Non-aqueous titration is a method used to determine the concentration of substances in solvents that do not contain water, allowing for the titration of weak acids and bases without interference from water's acid-base properties. Various types of non-aqueous solvents, including aprotic and protogenic solvents, are utilized, and specific indicators are employed to signal the endpoint of titrations. The document provides detailed procedures for preparing and standardizing non-aqueous titrants such as perchloric acid and methoxide, along with assay methods for compounds like sodium benzoate and ephedrine hydrochloride.