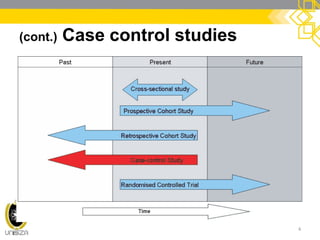
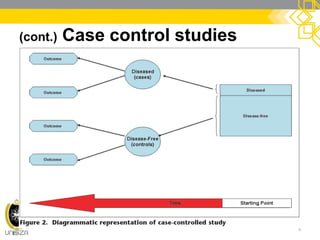
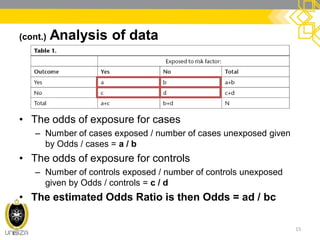
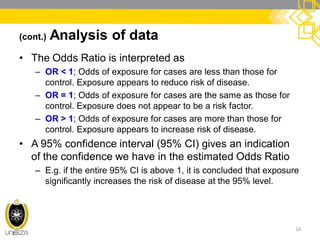
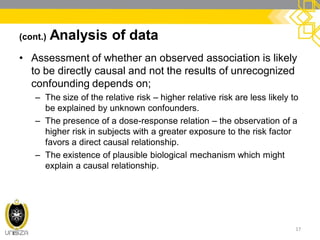
This document discusses case-control study designs. It defines a case-control study as one that establishes associations between exposures and disease by collecting risk factor information retrospectively from cases (people with the disease) and controls (people without the disease). It notes that careful selection of representative cases and controls is important to minimize bias. The document also describes how odds ratios are used to analyze relationships between exposures and outcomes in case-control studies and outlines both the advantages of case-control studies in examining rare diseases quickly and cheaply, as well as their limitations, such as difficulty establishing causality.