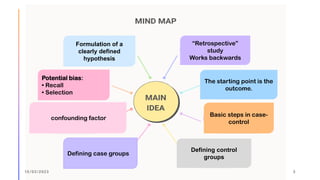
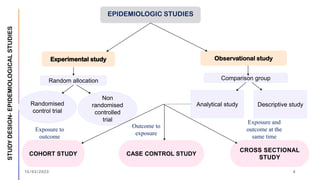
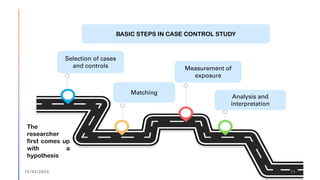
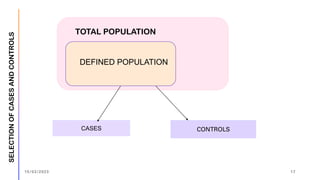
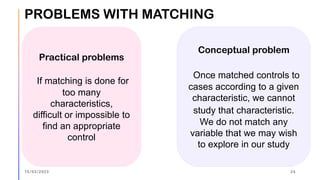
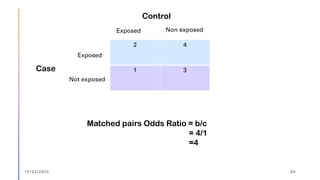
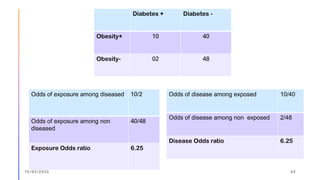
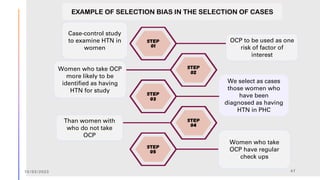
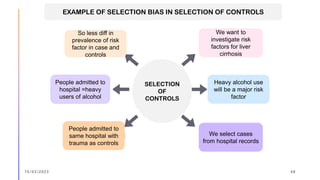
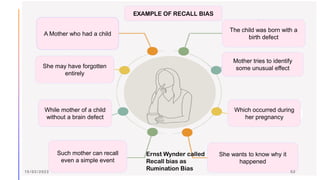
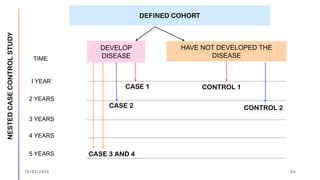
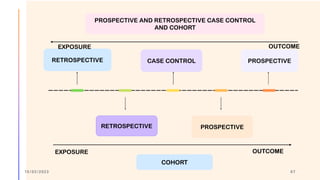
The document outlines the fundamentals of case-control studies in epidemiology, including their design, distinctive features, and basic steps such as selection of cases and controls, matching, and measurement of exposure. It discusses potential biases like recall and selection bias and highlights the advantages of conducting case-control studies, such as their efficiency and suitability for rare diseases. Additionally, the document covers the calculation and interpretation of odds ratios to determine associations between exposures and diseases.