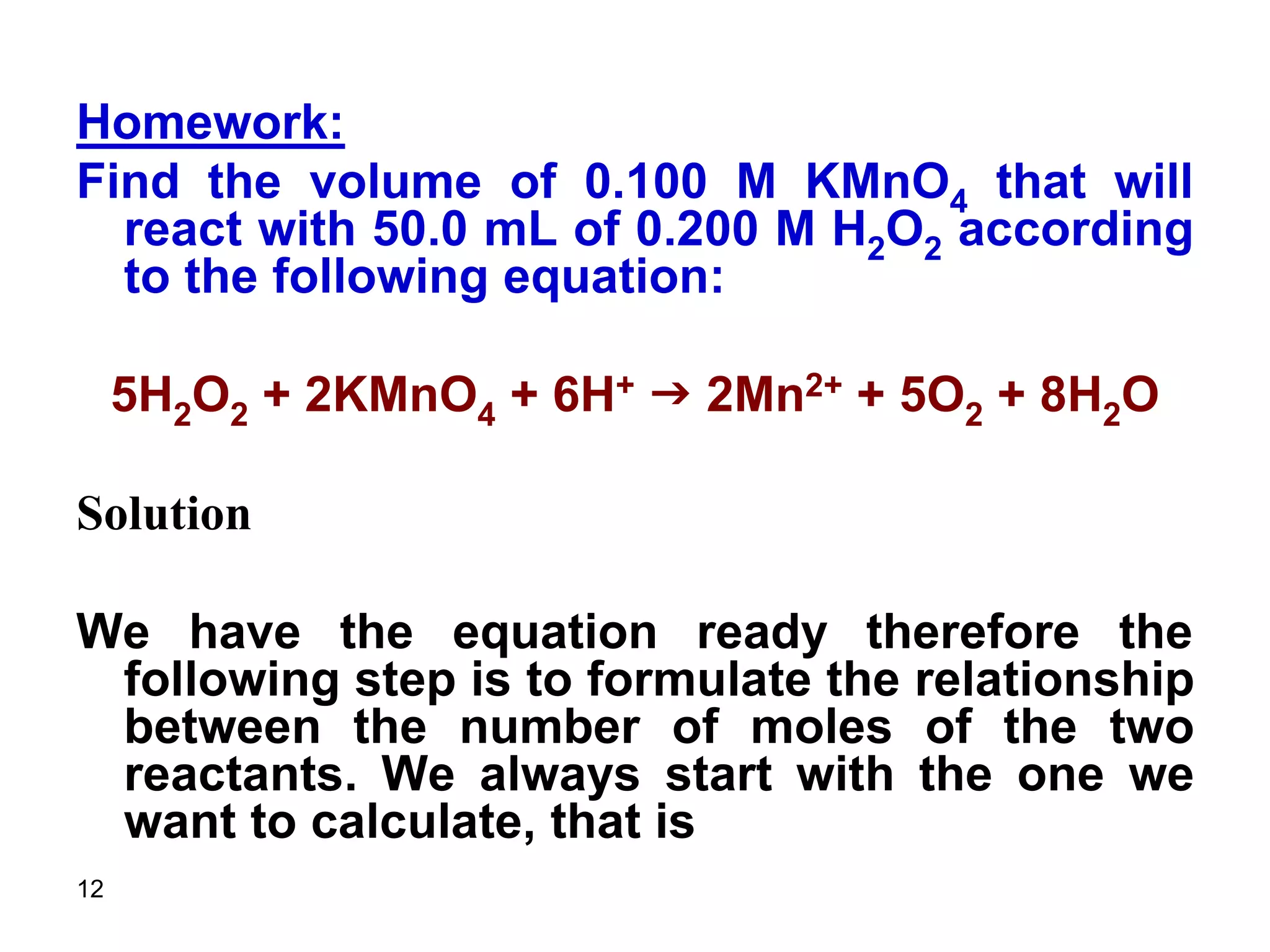
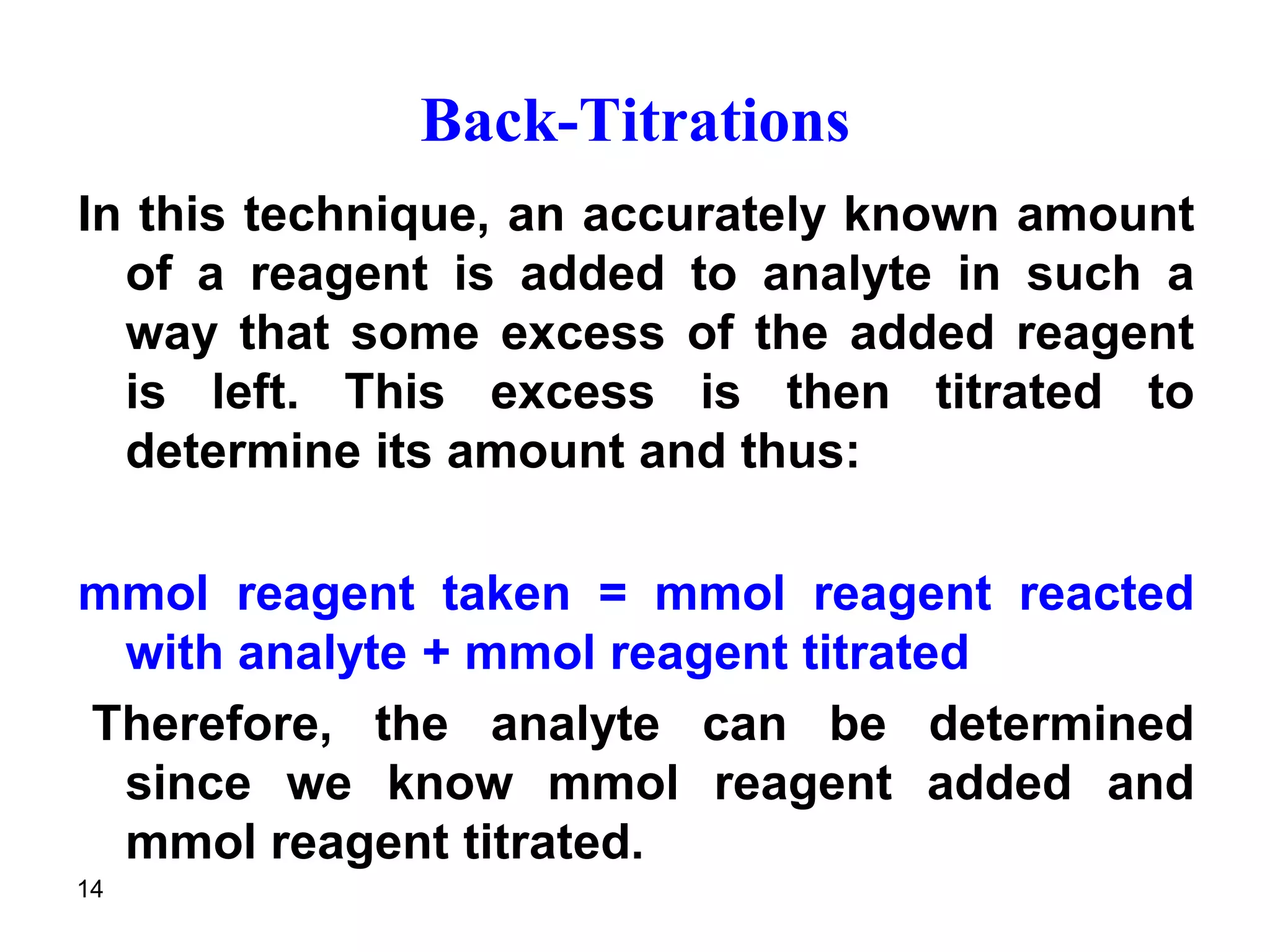
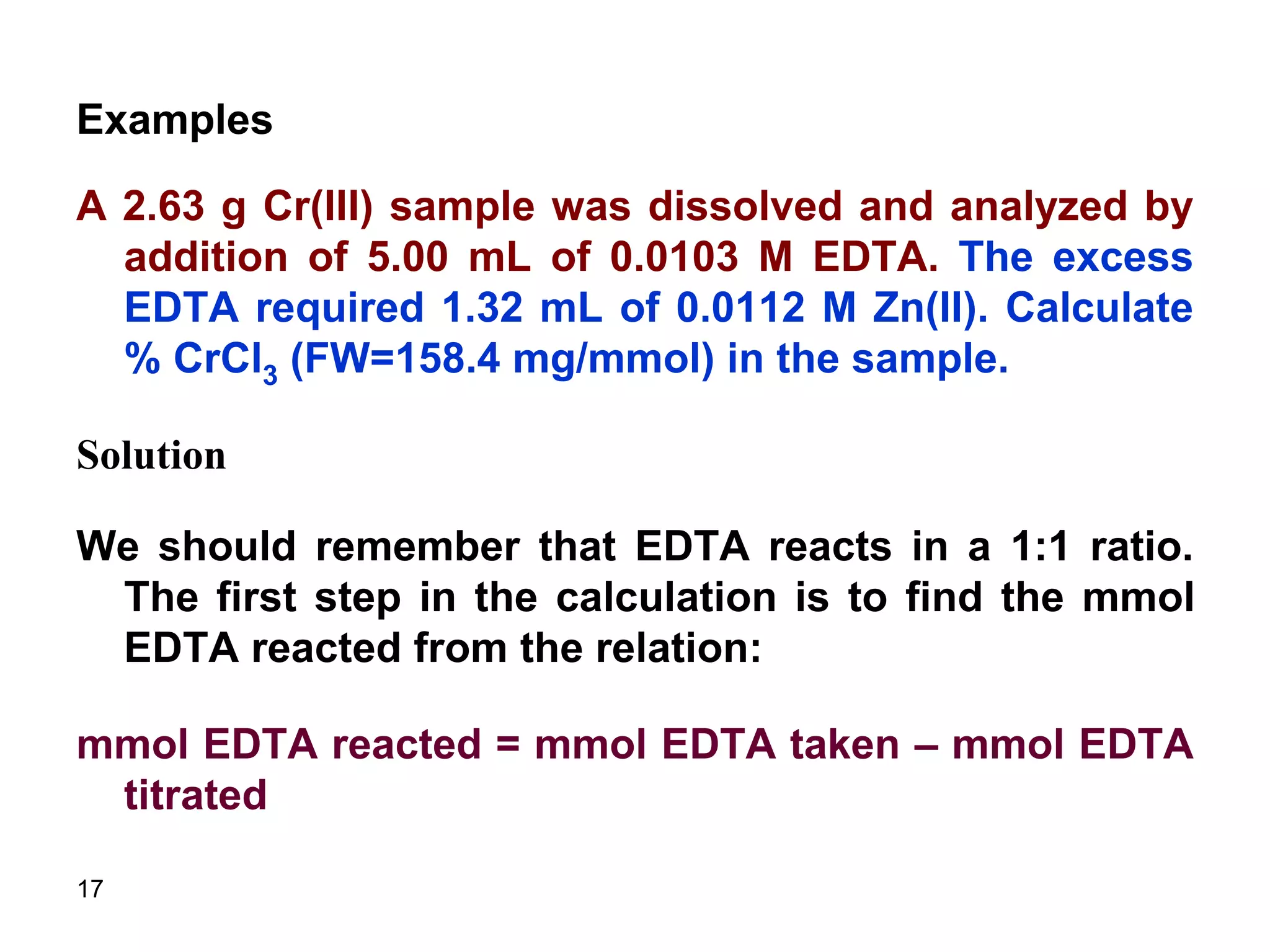
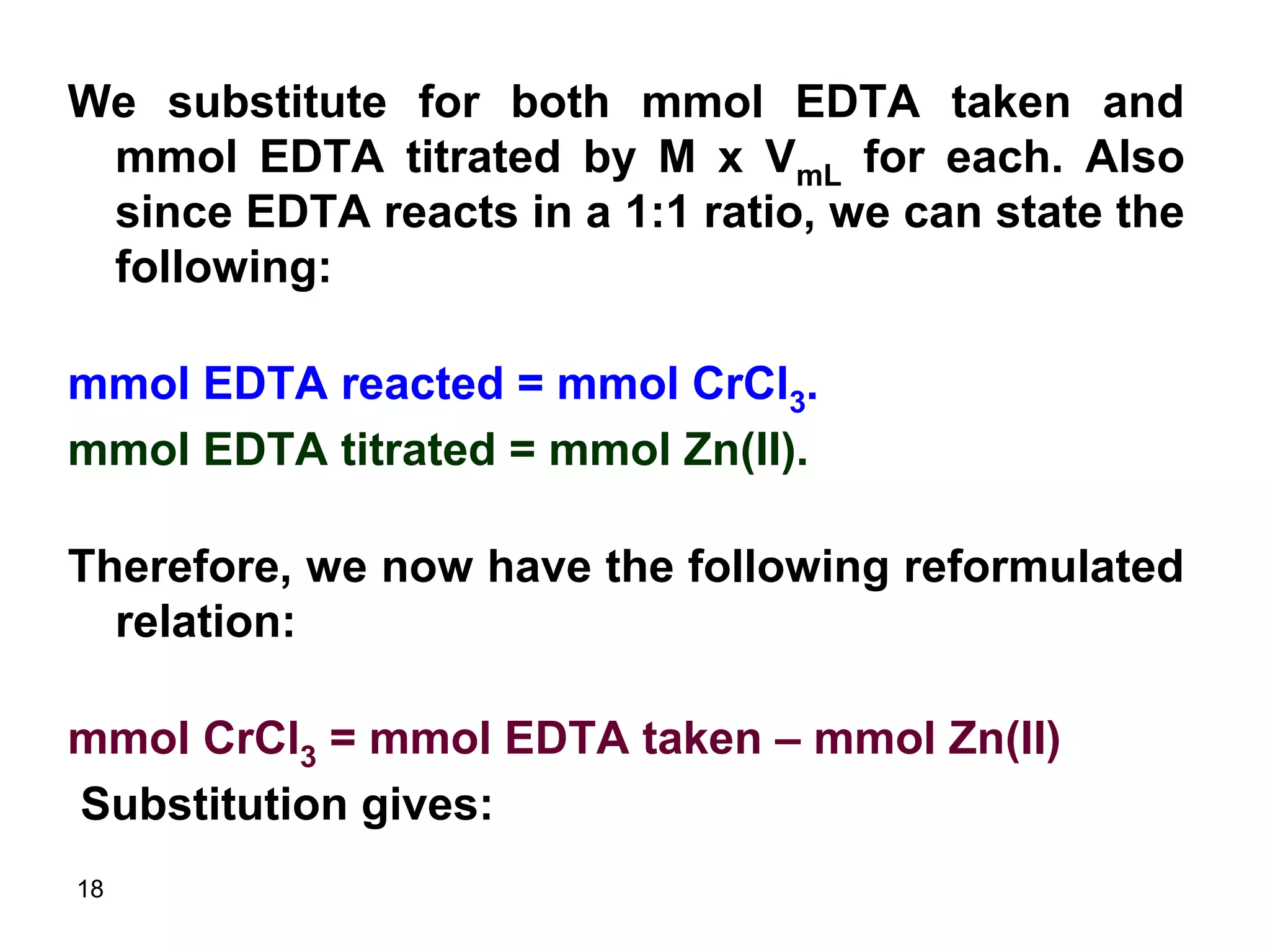
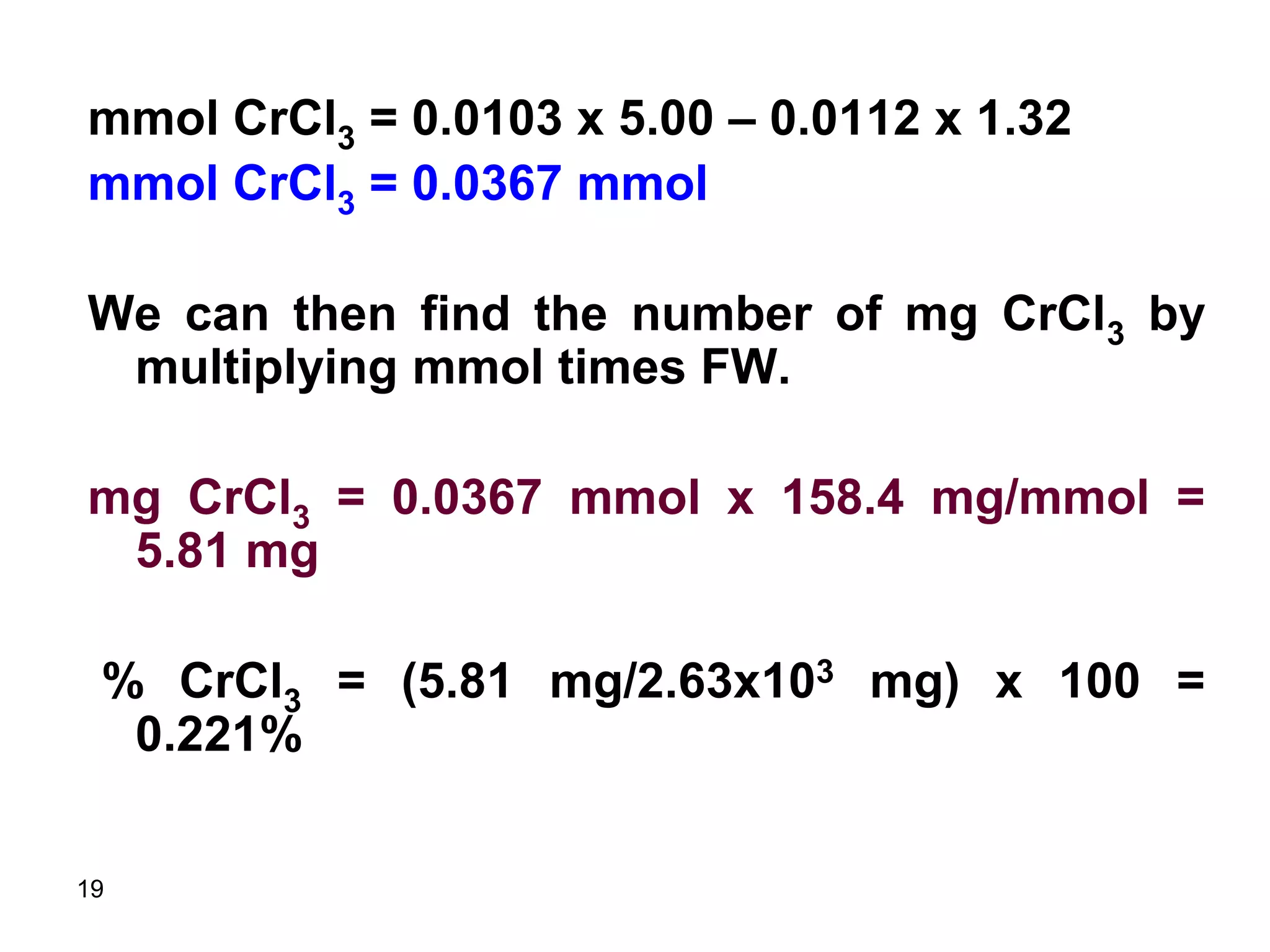
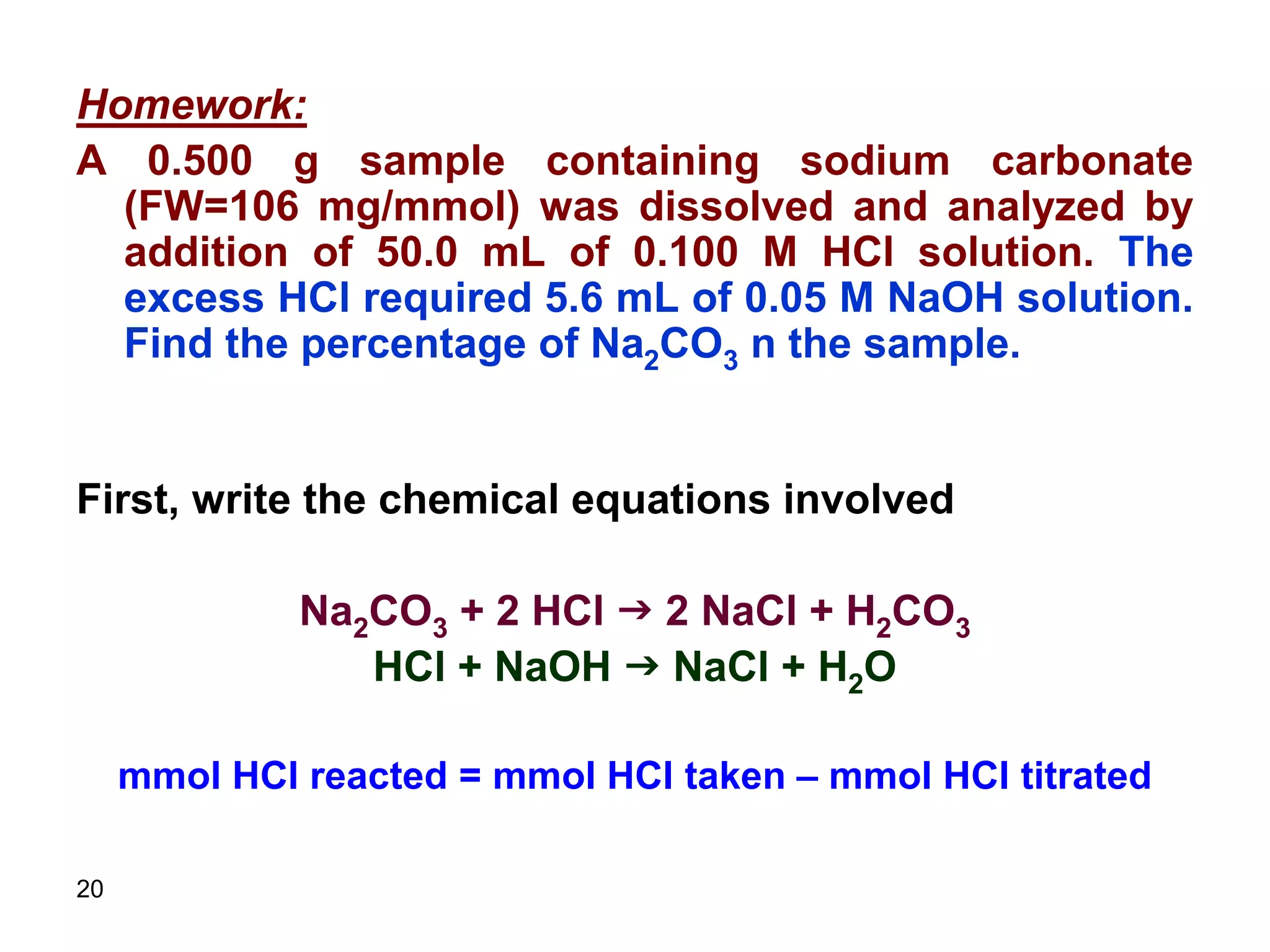
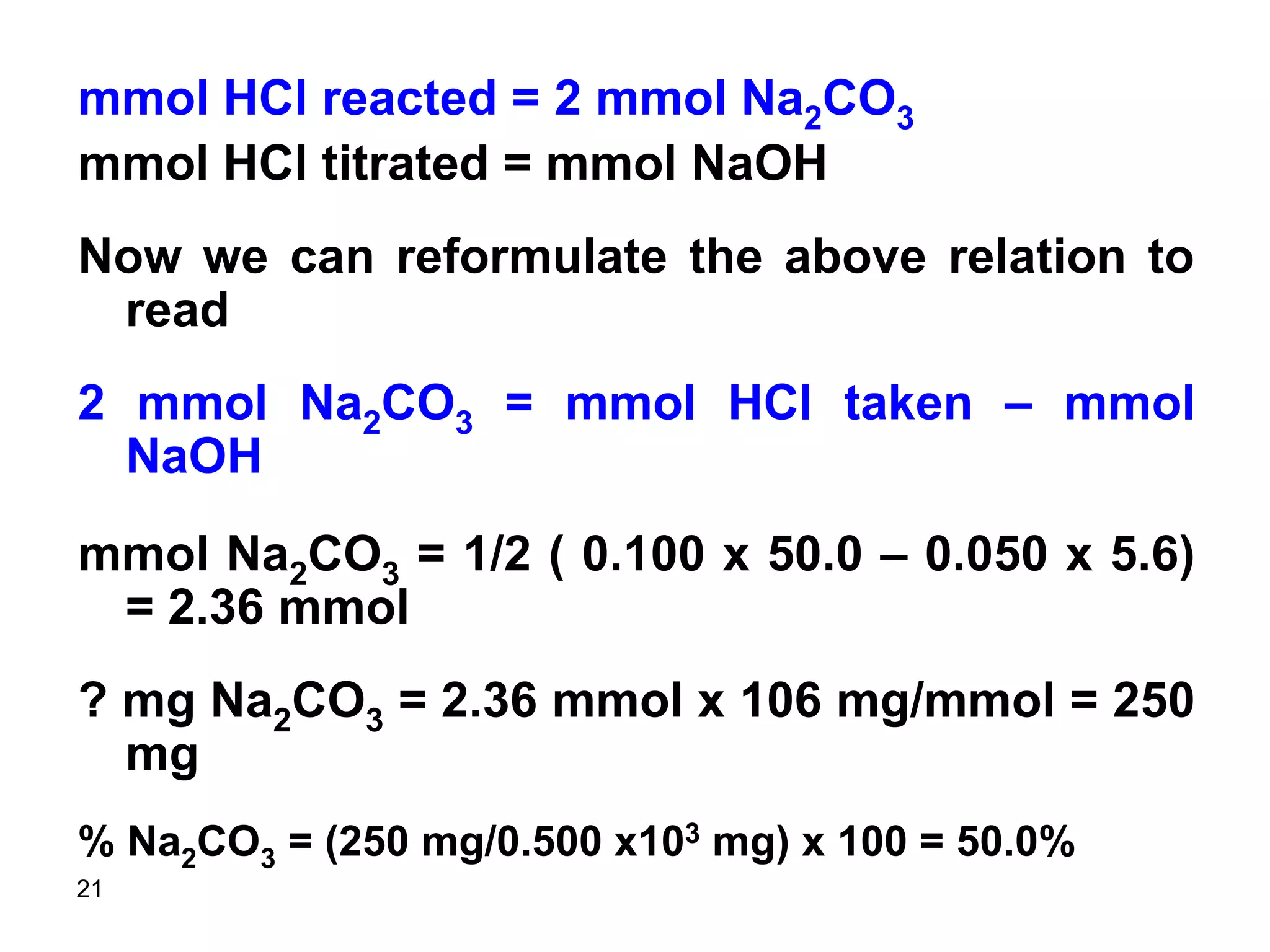
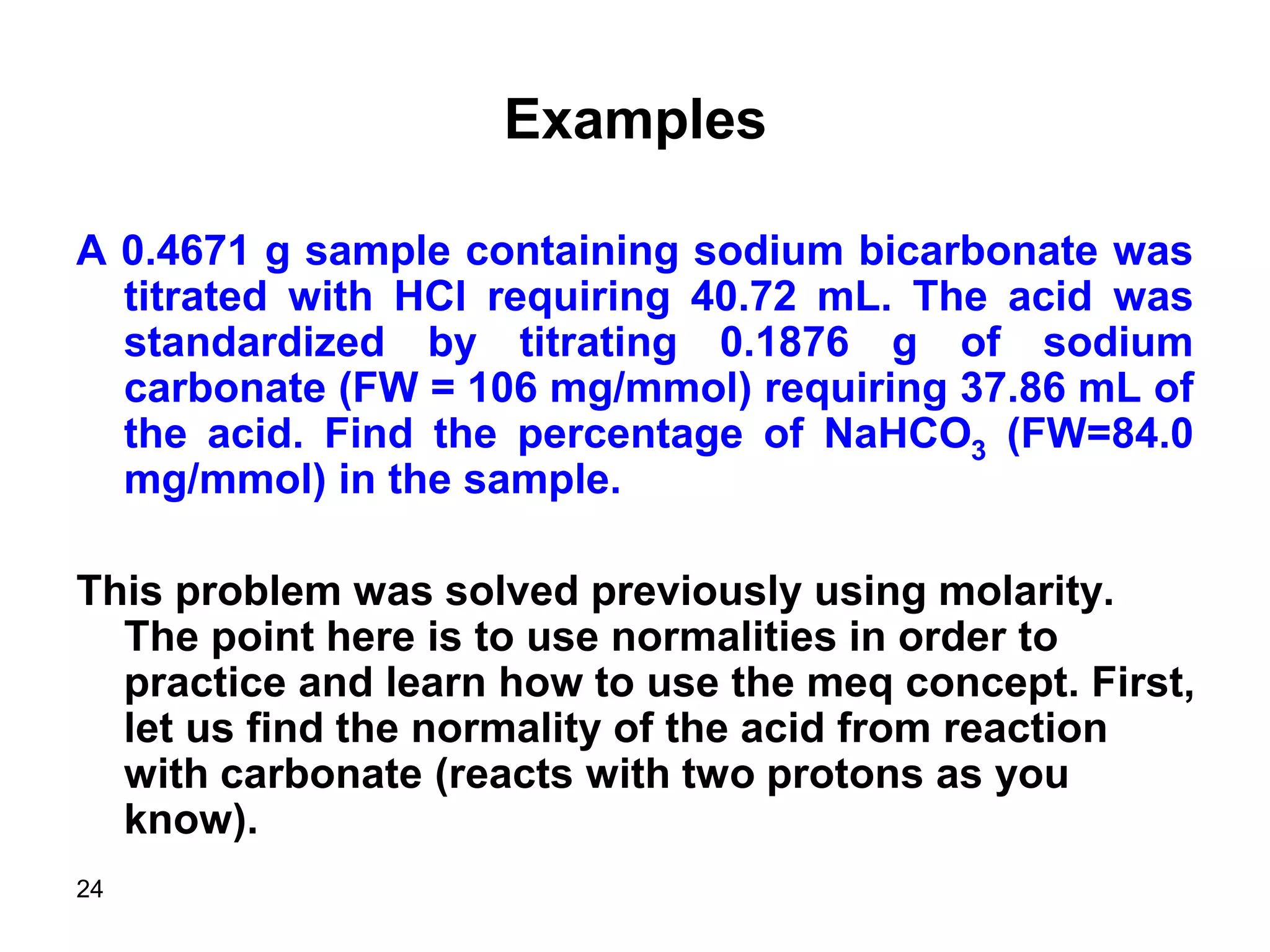
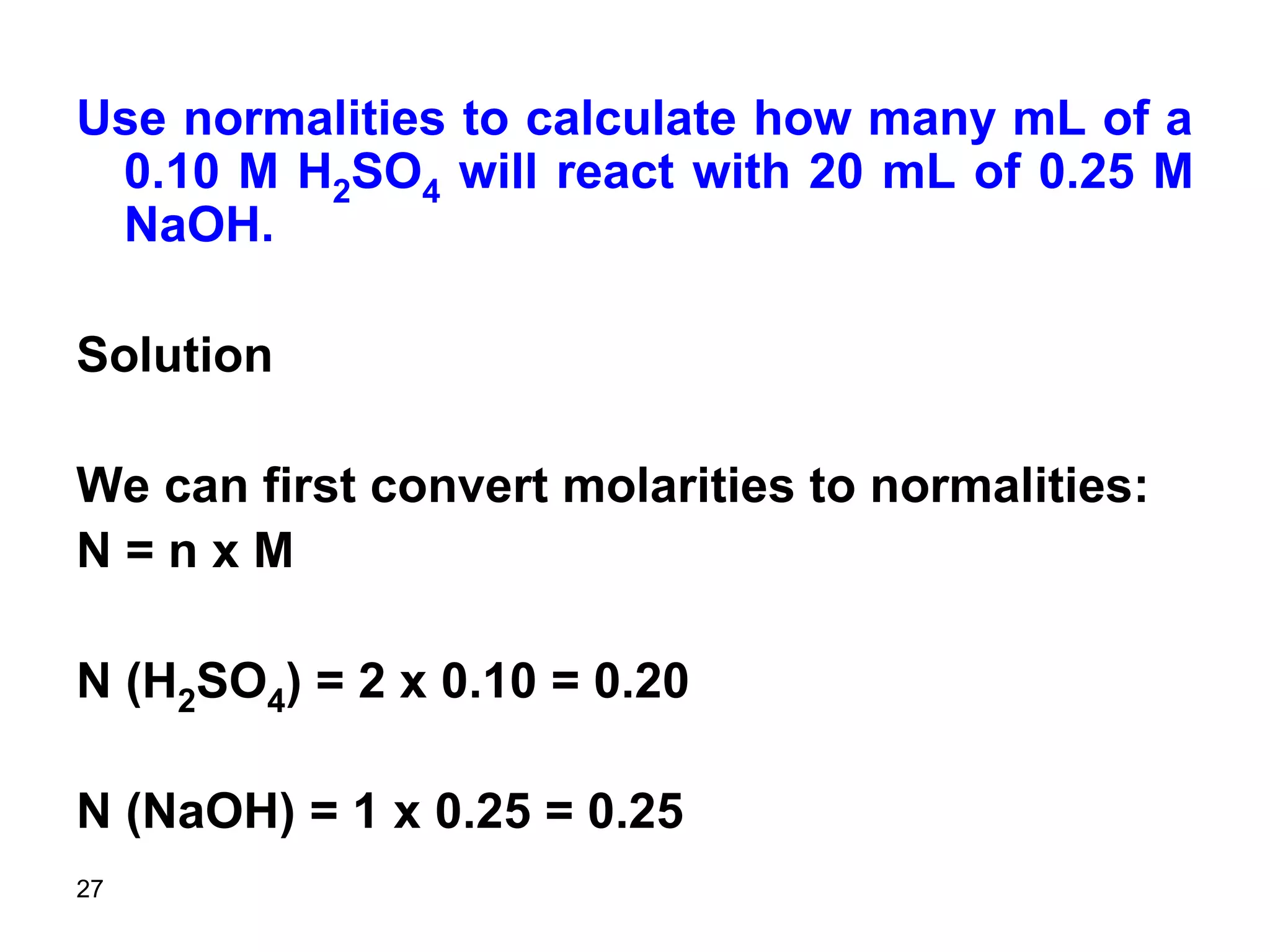
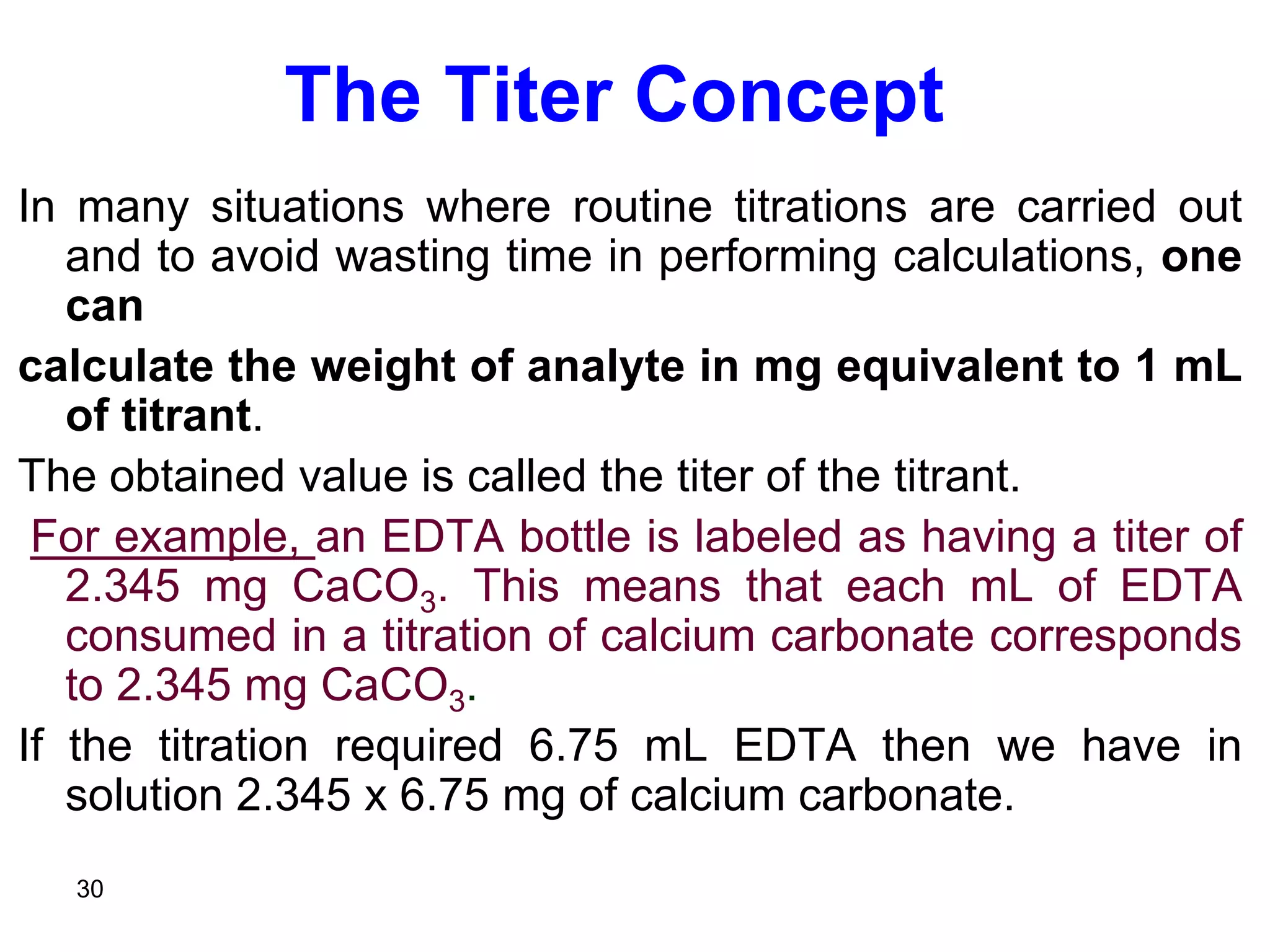
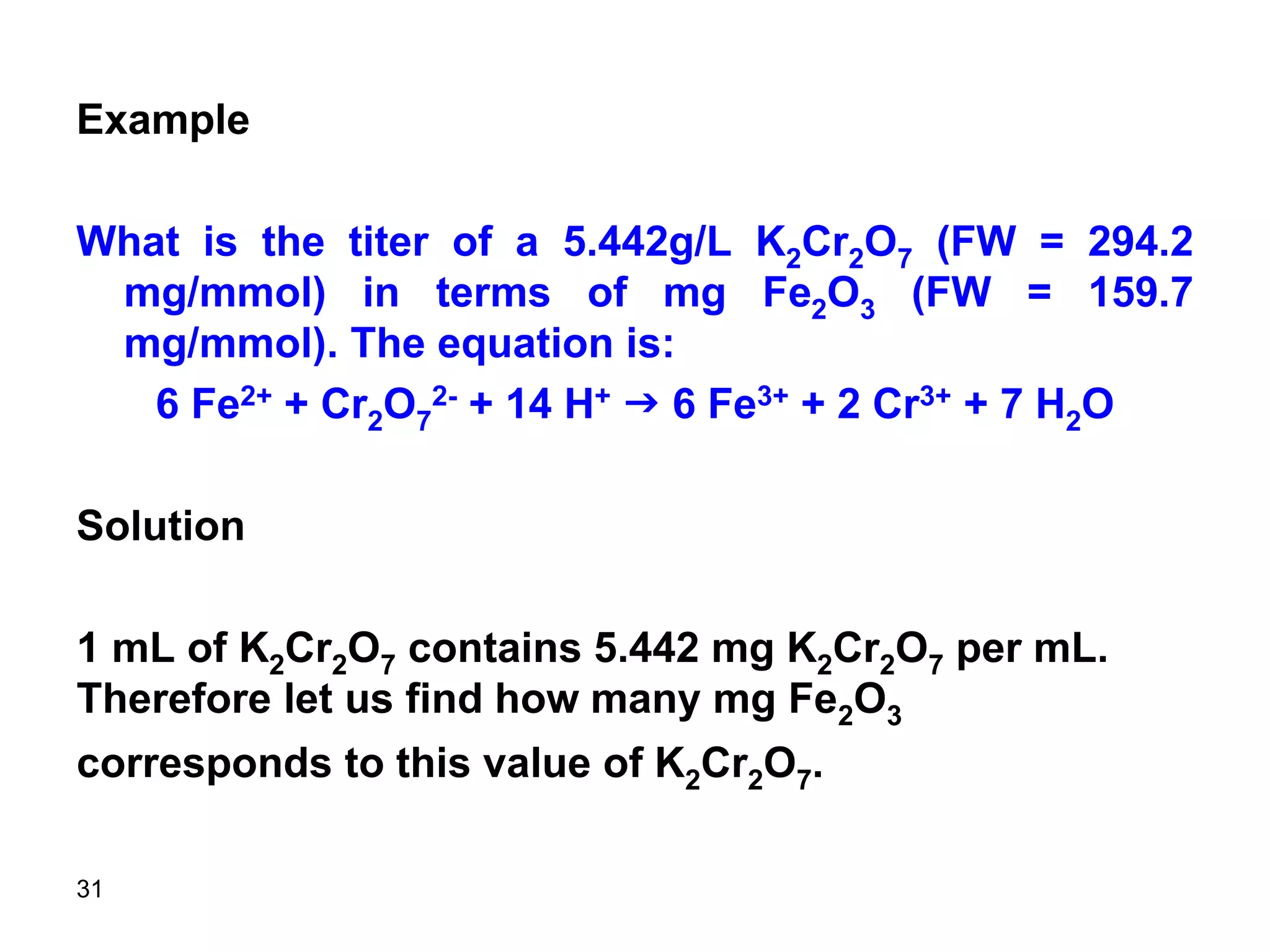
This document discusses stoichiometric calculations for volumetric analysis and titrations. It provides characteristics that reactions used for titrations should satisfy, such as having a known stoichiometry and being quantitative. It also discusses standard solutions, back titrations, and calculations using molarity and normality. Examples are provided for calculating unknown concentrations based on titration data and balanced chemical equations.






![10
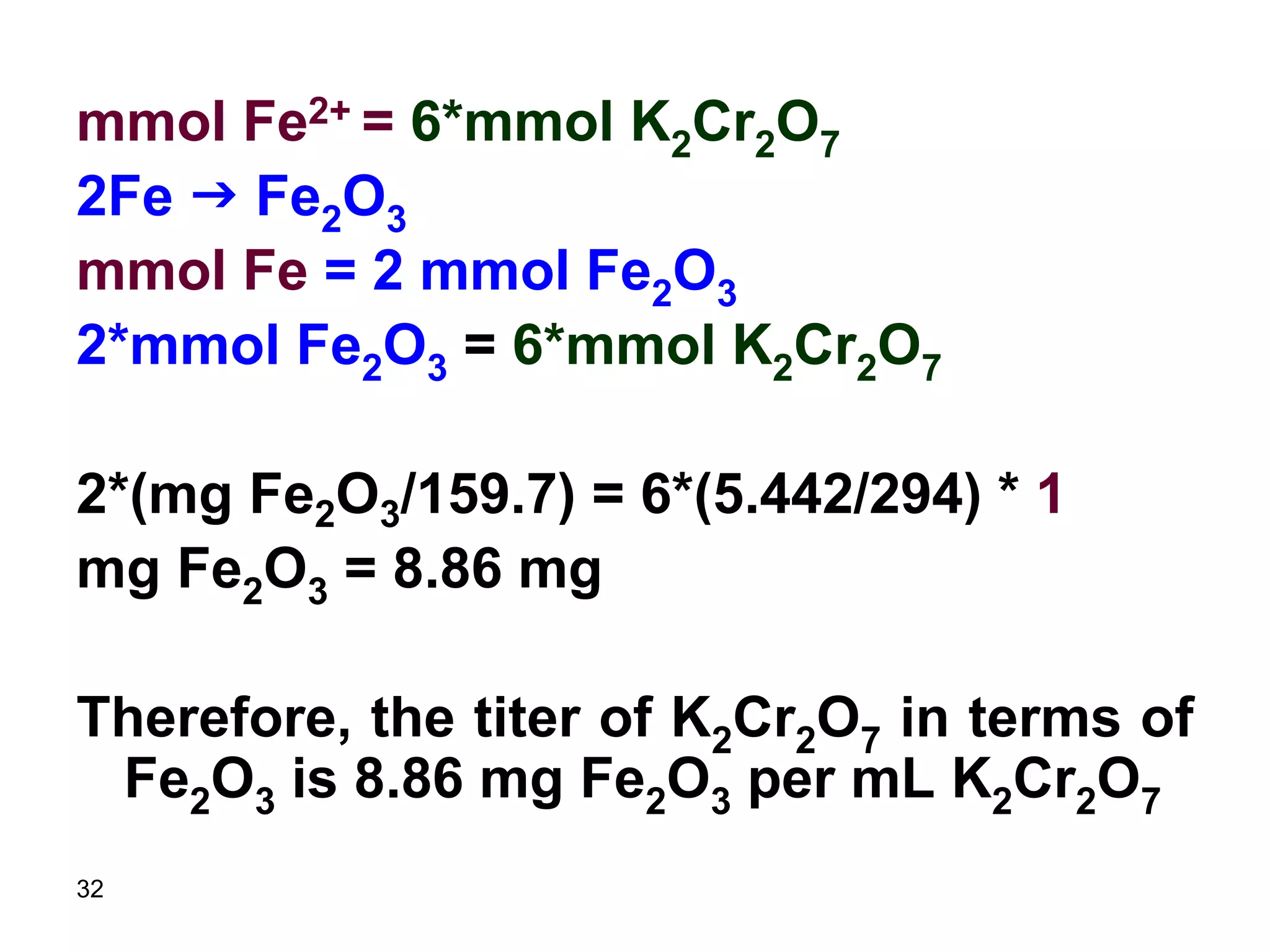
[mg Fe/(55.8 mg/mmol)] = 5 x (0.0206
mmol/mL) x 40.2 mL
mg Fe = 231 mg
This can also be done in a single step as
follows:
? mg Fe = (0.0206 mmol KMnO4 /mL) x
40.2 mL x (5 mmol Fe/mmol KMnO4) x
(55.8 mg Fe/mmol Fe) = 231 mg](https://image.slidesharecdn.com/stoichiometric-calculationspart2-230417211604-1a7203e9/75/Stoichiometric-calculations-part-2-ppt-10-2048.jpg)
![11
To calculate the mg Fe2O3 we set the following:
2Fe g Fe2O3
mmol Fe = 2 mmol Fe2O3
Substitute for mmol Fe in equation 1
2* [mg Fe2O3/ (159.7 mg/mmol)] = 5 x (0.0206 mmol/mL)
x 40.2 mL
mg Fe2O3 = 331 mg](https://image.slidesharecdn.com/stoichiometric-calculationspart2-230417211604-1a7203e9/75/Stoichiometric-calculations-part-2-ppt-11-2048.jpg)