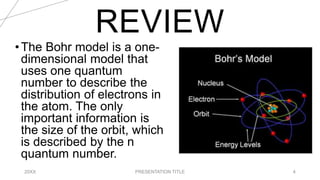
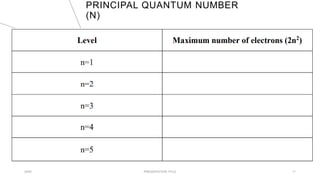
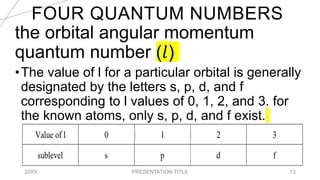
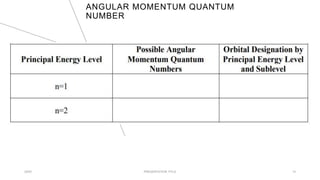
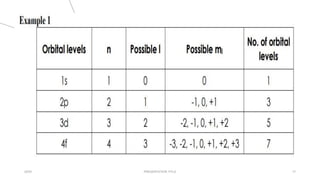
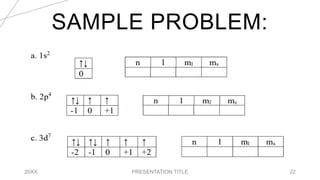
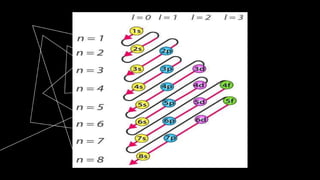
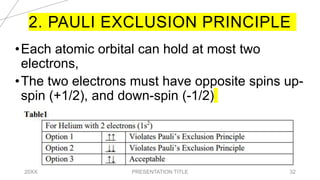
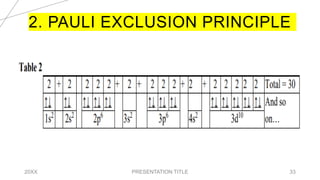
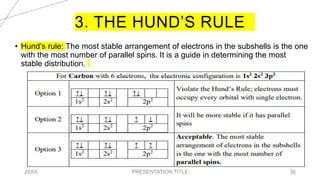
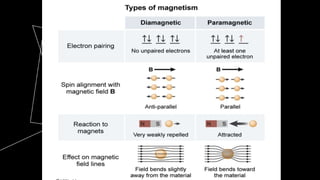
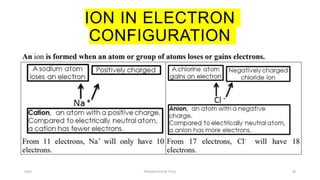
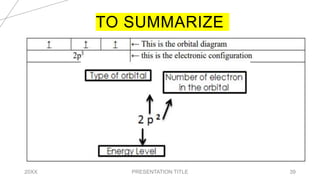
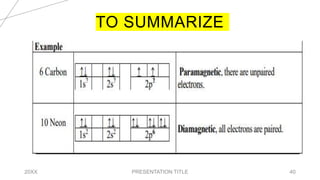
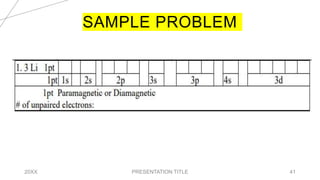
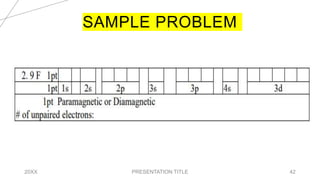
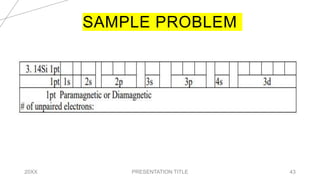
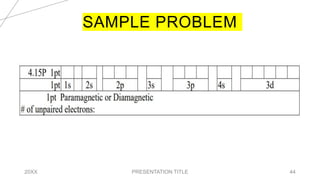
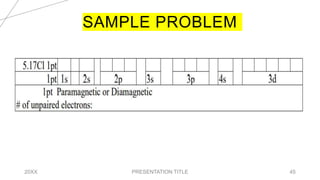
This document discusses quantum numbers and their use in describing an electron's state in an atom. It introduces the four quantum numbers - principal quantum number (n), orbital angular momentum quantum number (l), magnetic quantum number (ml), and electron spin quantum number (ms). Each quantum number provides a piece of information about the electron's properties, such as energy level, orbital shape, and spin. The values of the quantum numbers are constrained by certain rules. For example, the values of ml can range from -l to +l. The document also provides examples of writing out the full set of quantum numbers for some electron configurations.