Okay, let's solve this step-by-step:
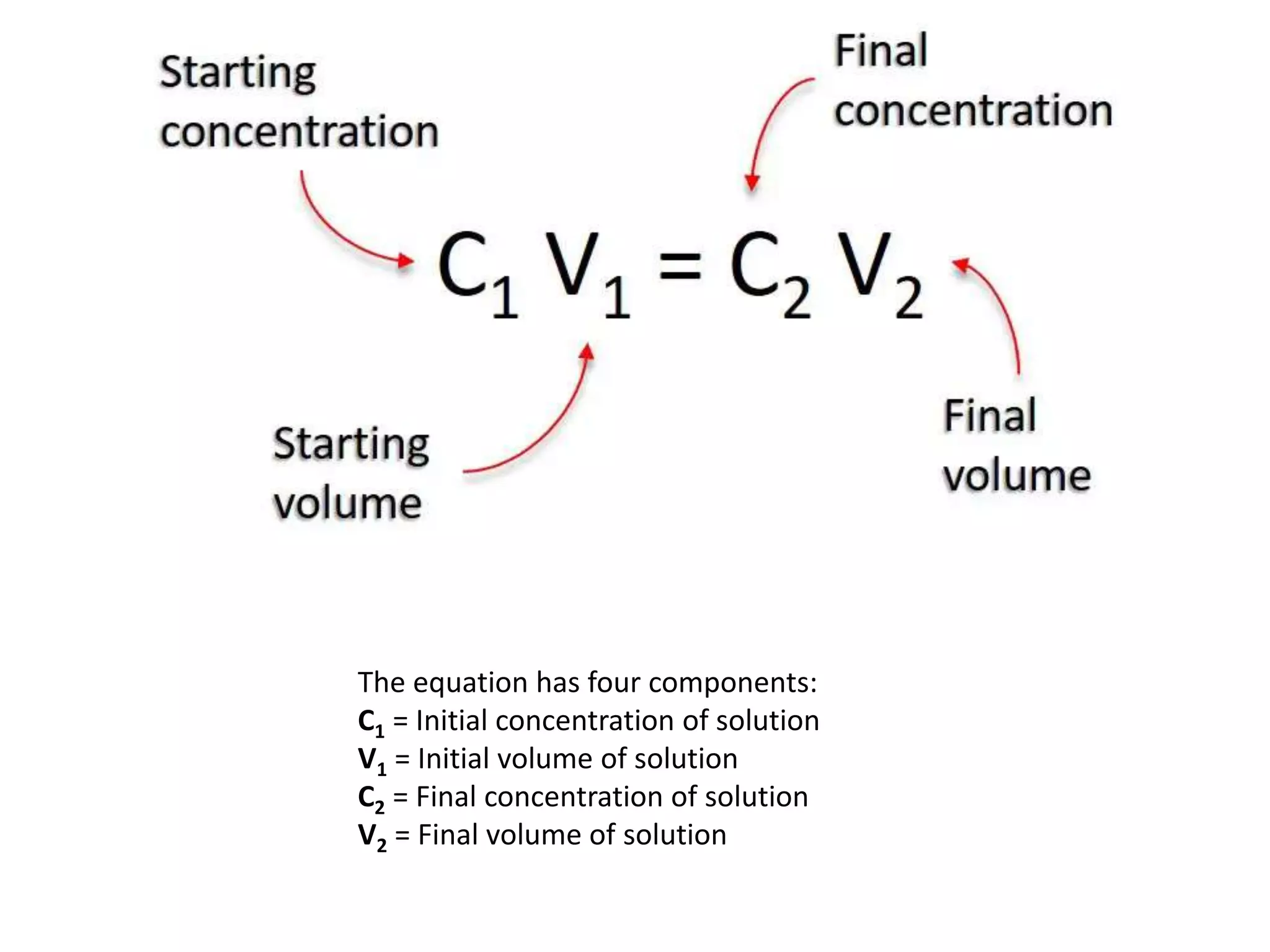
* Stock solution concentration (C1) = 1 M
* Stock solution volume (V1) = ?
* Required solution concentration (C2) = 0.1 M, 0.2 M, 0.5 M
* Required solution volume (V2) = 1 L = 1000 mL
Using the dilution formula: C1V1 = C2V2
For 0.1 M solution:
C1 = 1 M
C2 = 0.1 M
V2 = 1000 mL
Putting in the formula:
1M * V1 = 0.1M * 1000mL
V1 = 100