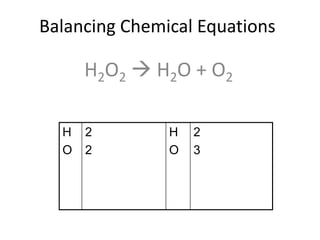
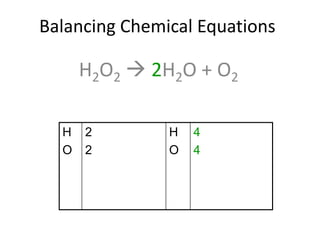
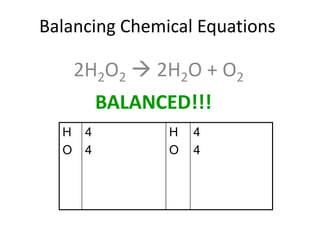
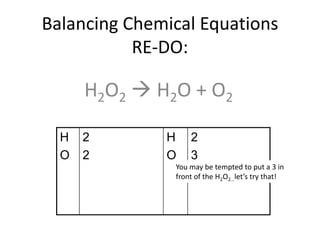
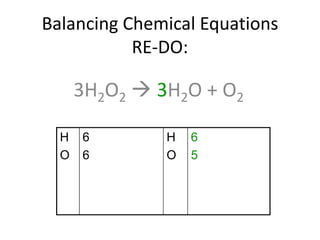
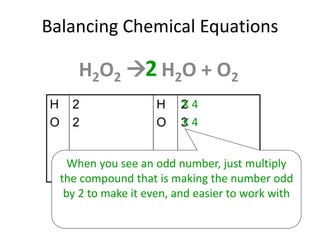
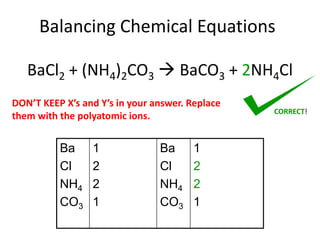
The document provides step-by-step instructions for balancing chemical equations. It demonstrates how to balance equations with monatomic and polyatomic ions, including making coefficients even numbers when needed. Examples show balancing equations such as H2O2 → H2O + O2, CaSO4 + KOH → Ca(OH)2 + K2SO4, and ZnS + O2 → ZnO + SO2. Key steps include writing element symbols, choosing coefficients, and balancing the numbers of each element on both sides of the equation.