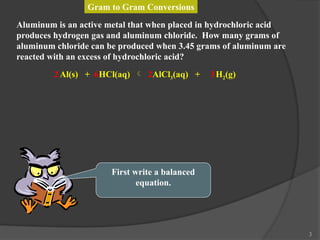
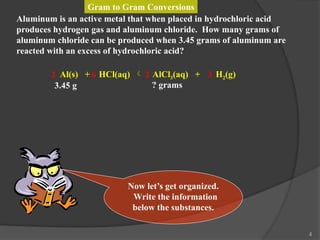
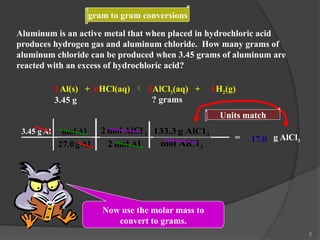
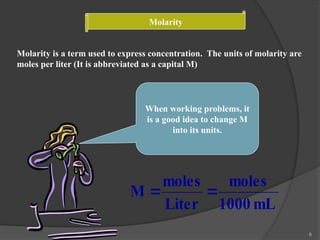
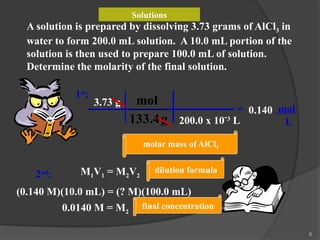
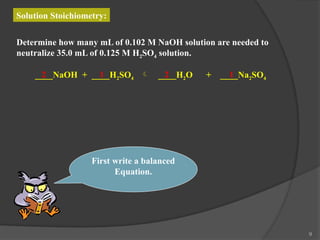
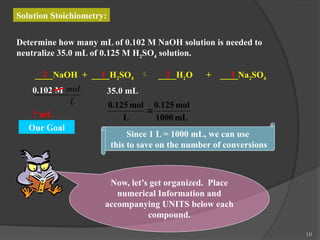
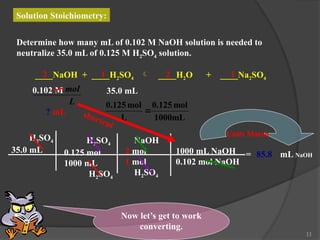
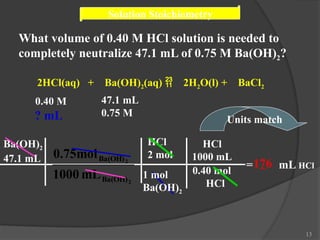
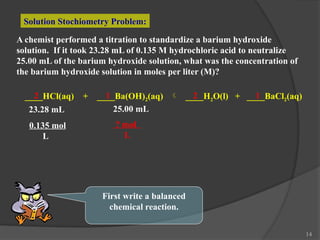
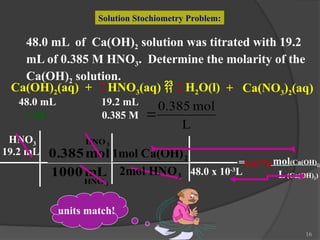
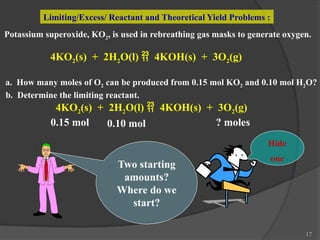
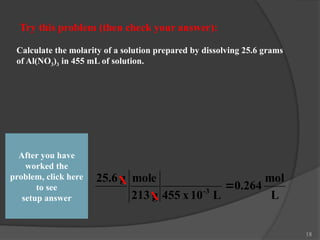
The document discusses solution stoichiometry, covering concepts such as the identification of compounds in a solution, writing balanced net ionic equations, and calculating moles of reactants and products. It provides examples of reactions involving aluminum and hydrochloric acid, explains molarity and dilution, and details methods for determining concentrations and neutralization volumes in acid-base reactions. Additionally, it includes problem-solving strategies for calculating theoretical yields and molarities in various chemical scenarios.