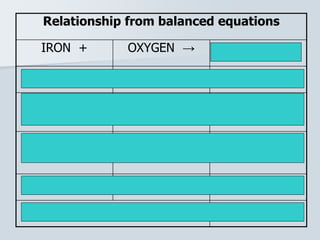
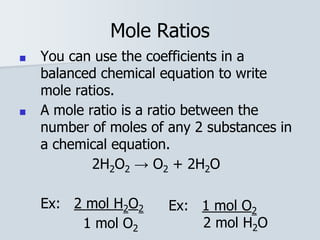
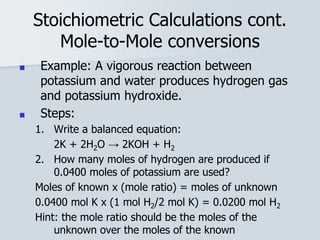
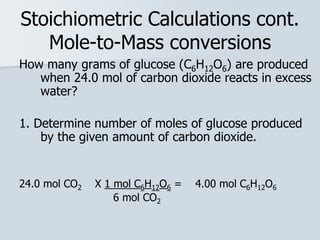
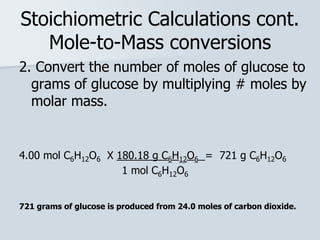
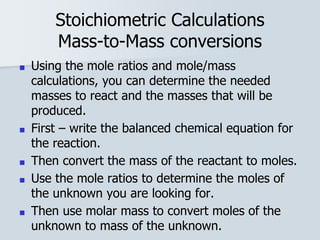
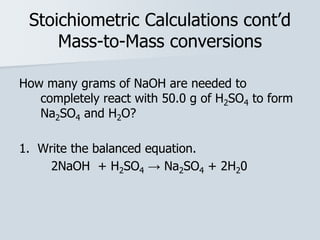
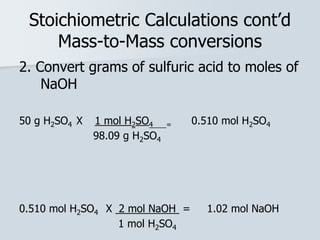
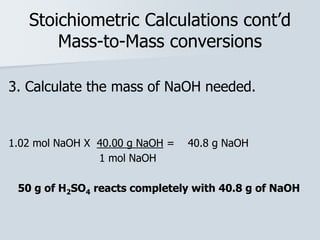
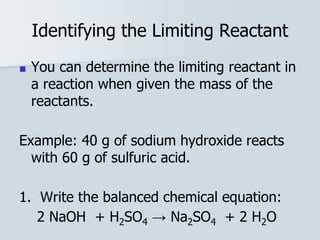
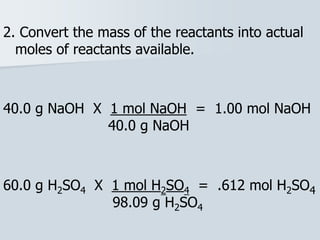
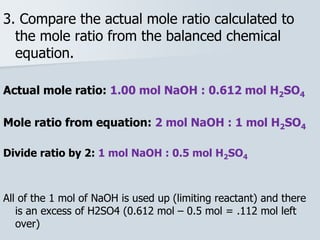
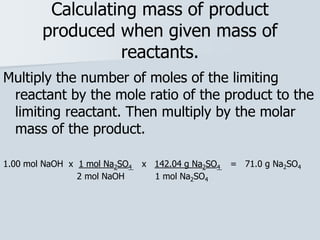
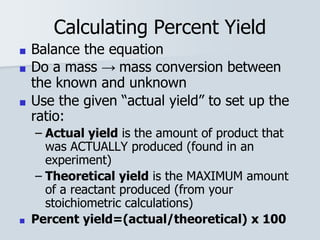
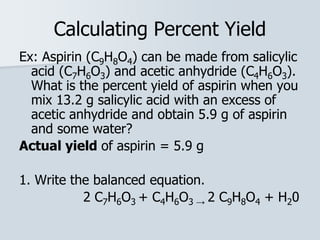
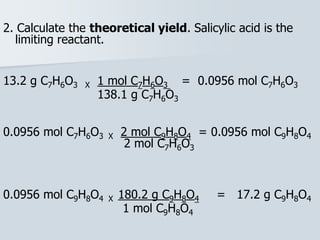
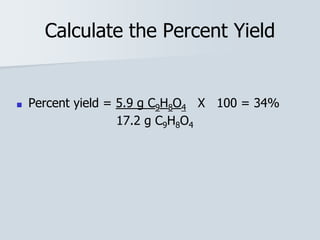
Stoichiometry is the quantitative relationship between reactants and products in a chemical reaction based on the law of conservation of mass. Balanced chemical equations show the mole ratios between substances and can be used to determine the amounts of reactants needed or products formed. Stoichiometric calculations allow conversion between moles and mass and identification of limiting reactants and theoretical versus actual yields of products.