The document discusses the properties and discovery of noble gases. It notes that:
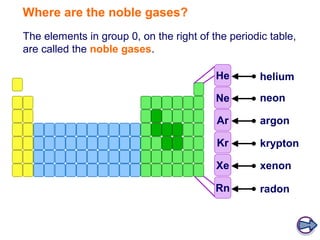
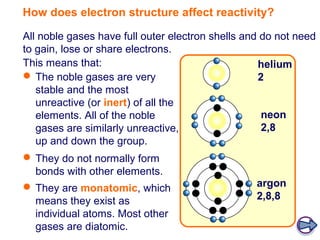
- Noble gases (group 0) are very unreactive due to their full outer electron shells. This stability makes them similar across the group.
- Helium was first discovered on the sun in 1868. Argon was discovered in the 1890s after nitrogen from air was found to contain a small quantity of an inert element.
- Ramsay realized argon needed its own group and later discovered it contained traces of other noble gases - neon, krypton, and xenon. Radon was discovered in 1900.