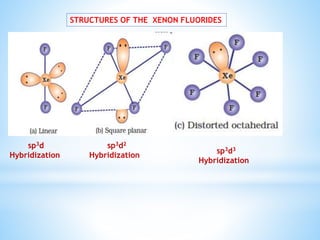
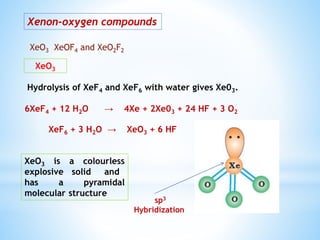
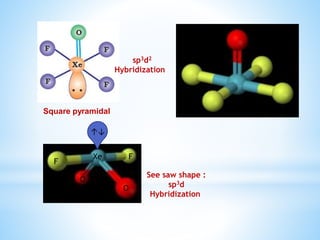
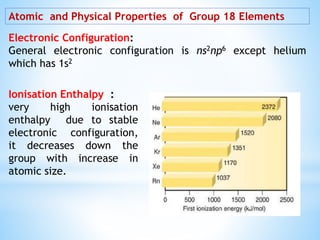
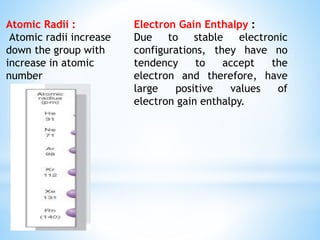
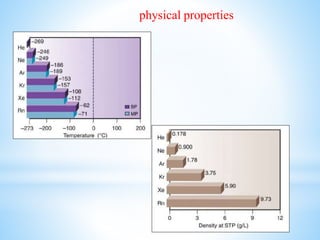
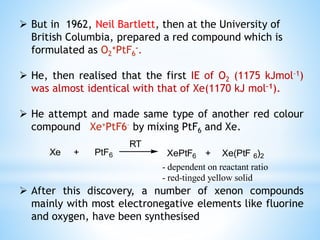
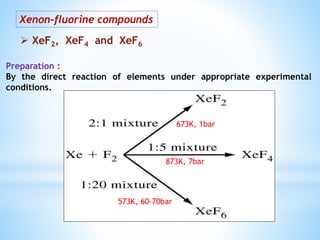
Group 18 of the periodic table contains the noble gases: helium, neon, argon, krypton, xenon, and radon. They are colorless, odorless gases that are chemically unreactive due to their stable electronic configurations. While traditionally considered inert, xenon can form some compounds with highly electronegative elements like fluorine and oxygen. Helium has important uses including in balloons, cooling nuclear reactors, and medical MRI machines due to its unique properties. Other noble gases like neon and argon are used for lighting and providing inert atmospheres.






![ Xenon fluorides react with fluoride ion acceptors to
form cationic species and fluoride ion donors to form
fluoroanions.
XeF2 + PF5 → [XeF]+ [PF6]–
XeF4 + SbF5 → [XeF3]+ [SbF6]–
XeF6 + MF → M+ [XeF7]– (M = Na, K, Rb or Cs)](https://image.slidesharecdn.com/econtentpdfnoblegasesxenoncompounds-230211124127-6ba2cad4/85/Econtent_Pdf_NobleGasesXenonCompounds-pdf-12-320.jpg)