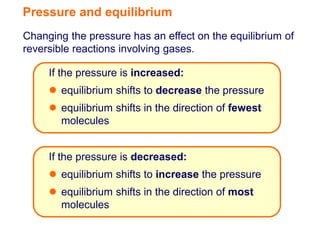
The document discusses how changing the pressure affects the equilibrium of reversible gas reactions. It states that if the pressure is increased, the equilibrium will shift in the direction that decreases the number of gas molecules. If the pressure is decreased, the equilibrium will shift in the direction that increases the number of gas molecules. It also provides the example of nitrogen dioxide and dinitrogen tetroxide, explaining that if the pressure of this system is increased, more dinitrogen tetroxide will be produced.