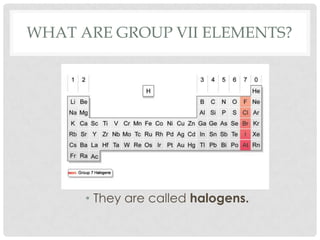
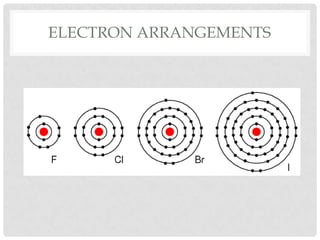
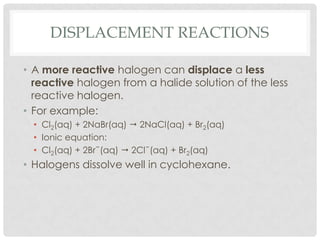
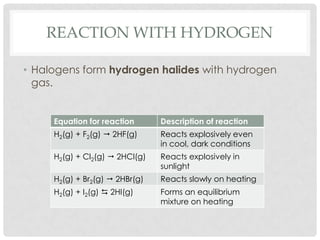
Group VII elements are called halogens. They exist as diatomic molecules (F2, Cl2, Br2, I2) and have seven electrons in their outer shell. Fluorine has the smallest atomic radius while iodine has the largest due to more electron shells. Melting and boiling points decrease from fluorine to iodine due to weaker van der Waals forces between larger molecules. Electronegativity decreases from fluorine to iodine as the nucleus attracts electrons less. Halogens can gain electrons to form ions or share electrons to form covalent bonds. More reactive halogens can displace less reactive ones from solutions.