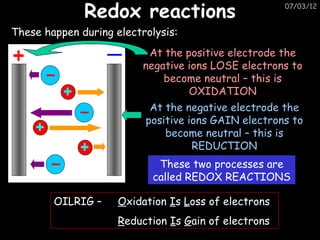
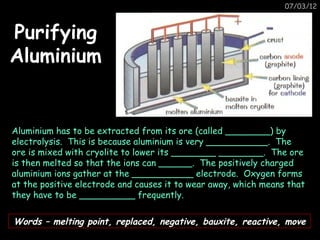
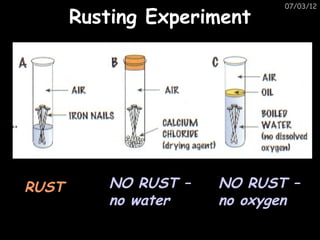
The document discusses the reactivity and reactions of various metals, including:
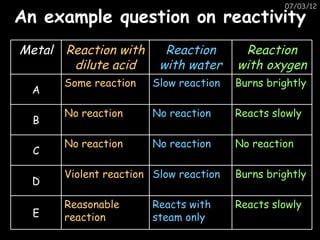
- Metals react with oxygen to form metal oxides, and this reaction can be sped up by burning the metal.
- Metals react with water and acids to form metal hydroxides/oxides or salts and release hydrogen gas.
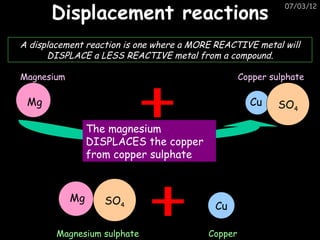
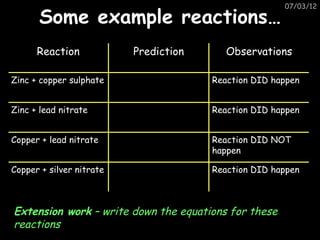
- More reactive metals can displace less reactive metals from compounds in a displacement reaction.
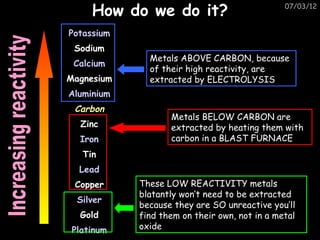
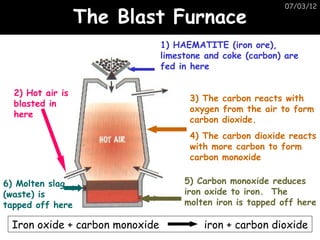
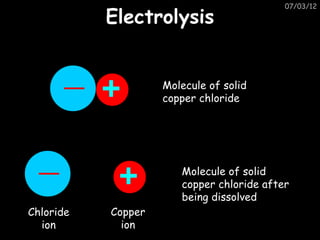
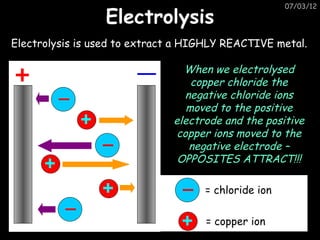
- Metals are extracted from their ores using electrolysis or heating with carbon in a blast furnace.