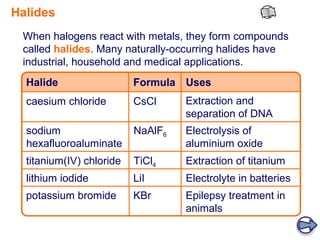
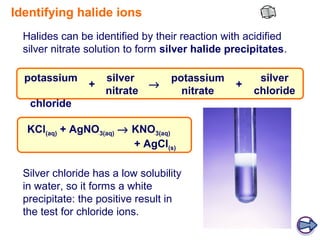
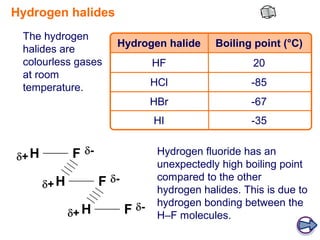
Halides are compounds formed between halogens (F, Cl, Br, I) and metals. They have many industrial, medical, and household applications. Halides can be identified by their reaction with silver nitrate solution to form precipitates of insoluble silver halides. The hydrogen halides are colorless gases with hydrogen fluoride having an unexpectedly high boiling point due to hydrogen bonding between H-F molecules. Larger halide ions are more reactive reducing agents as their outer electrons are farther from the nucleus and more easily donated.