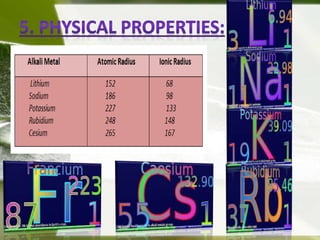
Alkali metals have a single outer electron, making them highly reactive and electropositive. They are never found naturally in their elemental forms, instead occurring as oxides, halides, borates, silicates, and nitrates. Alkali metals increase in size down the group and have low ionization energies, with lithium being the smallest and most reactive and cesium being the largest and least reactive. They react vigorously with air, water, halogens, and dihydrogen to form ionic compounds. Common uses include softening hard water, manufacturing glass, detergents, and as reducing agents.











![(vi) Solutions in liquid ammonia:
The alkali metal dissolves in
liquid ammonia giving deep blue
solutions which are conducting
in nature.
M + (x + y)NH3
[M(NH3)x]+ +
[e(NH3)y]-
The solutions are paramagnetic
and on standing slowly librates
hydrogen resulting in the
formation of amide.
M+
(am) + e- +NH3(l) MNH2(am) +
1/2H2(g)
(where ‘am’ denotes solution in
ammonia.)](https://image.slidesharecdn.com/chemistryproject1-141204080117-conversion-gate02/85/Alkali-metals-13-320.jpg)





