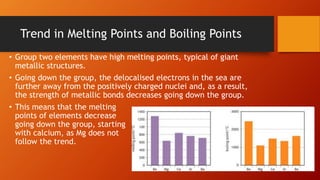
Group 2 elements, known as alkaline earth metals, exhibit increasing atomic radius and reactivity with water down the group, with magnesium and calcium reacting differently with steam and cold water. The solubility of hydroxides increases going down the group while sulfate solubility decreases, making barium sulfate virtually insoluble and useful in medical imaging. Additionally, magnesium is used in titanium extraction, and calcium oxide is involved in flue gas desulfurization, converting pollutants into calcium sulfate.