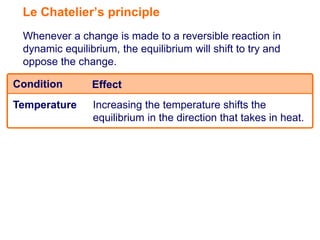
Le Chatelier's principle states that if a system in dynamic equilibrium experiences a change, the equilibrium will shift to counteract the change. Specifically, increasing temperature shifts equilibrium towards the endothermic direction, increasing concentration shifts equilibrium away from that substance, and increasing pressure shifts equilibrium away from the gas producing side.