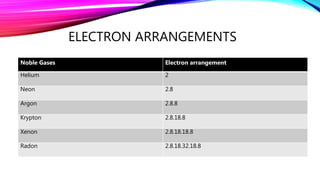
Group 18 of the periodic table consists of the noble gases: helium, neon, argon, krypton, xenon, and radon. These elements have full outer electron shells, making them very unreactive chemically. They have low densities, melting and boiling points, are colorless, and poor conductors of heat and electricity. Various noble gases have important industrial and medical uses, such as filling weather balloons, neon signs, light bulbs, lasers, cancer treatment, and more.