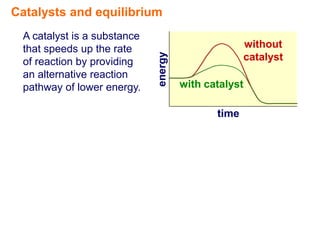
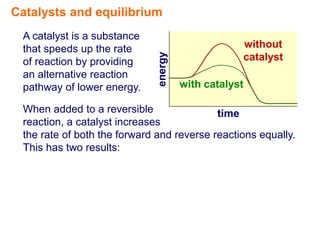
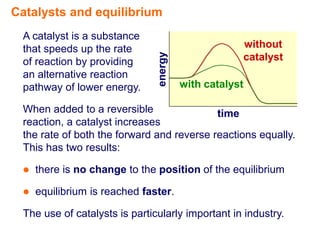
Catalysts speed up chemical reactions by lowering the activation energy of the reaction pathway, without being used up in the process. When added to a reversible reaction, a catalyst increases the rates of both the forward and reverse reactions equally, resulting in no change to the equilibrium position but equilibrium being reached faster. Catalysts are particularly important in industrial applications as they allow reactions to proceed more quickly.