The document discusses the rules and steps for writing chemical formulas:
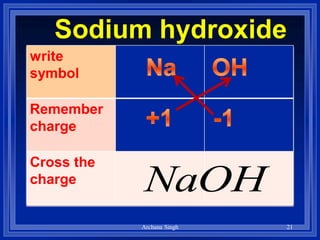
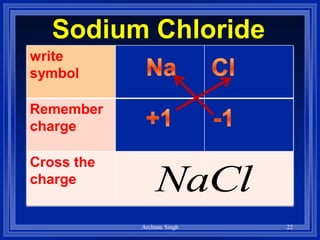
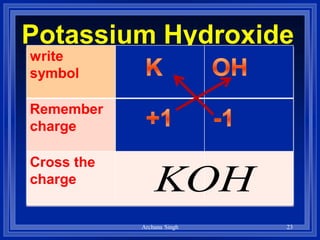
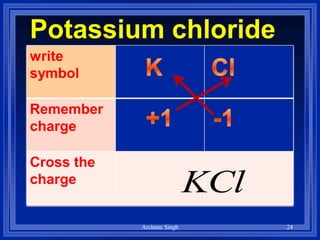
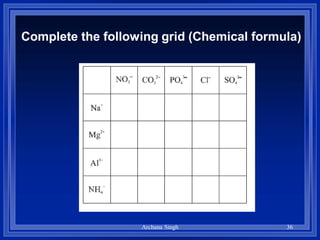
1) Formulas use symbols and subscripts to show which elements are present and how many atoms of each.
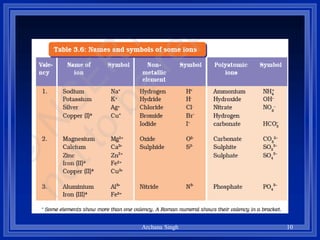
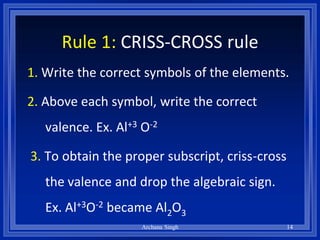
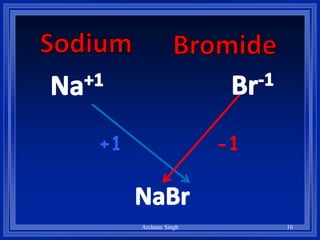
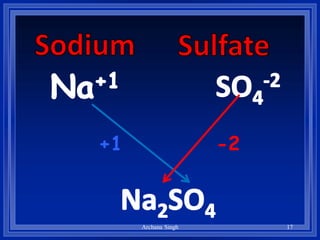
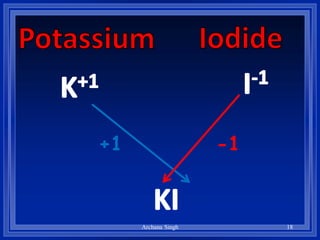
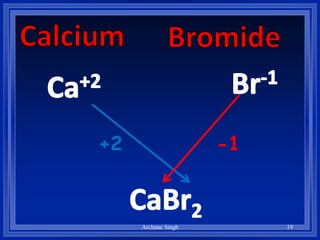
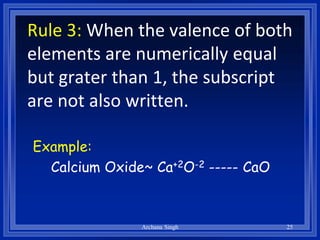
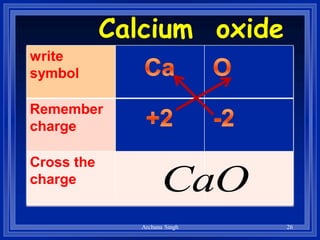
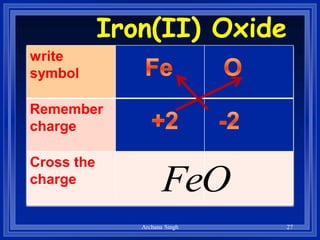
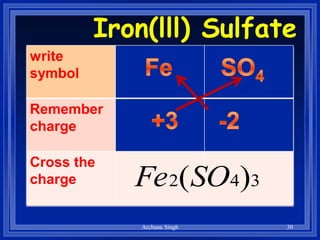
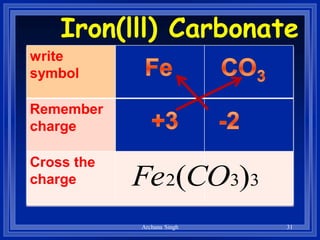
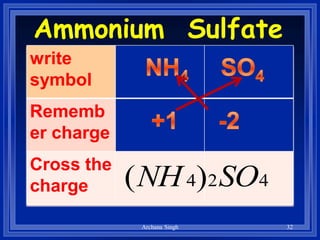
2) Rules include balancing charges by "criss-crossing" valence numbers, omitting subscripts of 1, and using parentheses for polyatomic ions.
3) The steps are to determine symbols and valences, write the positive element first, and balance charges to obtain the correct subscripts.