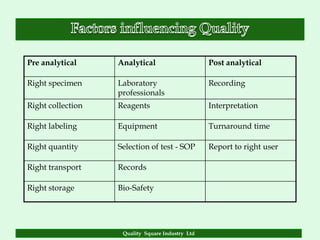
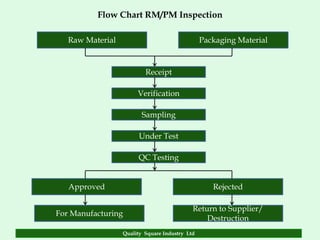
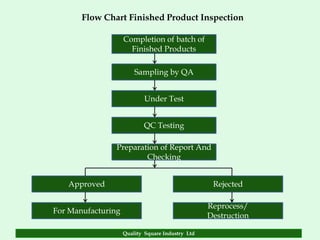
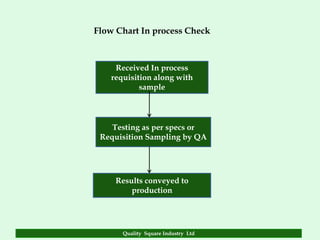
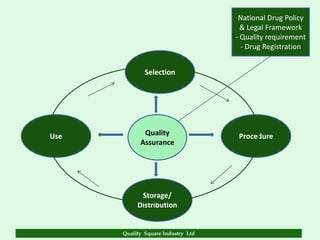
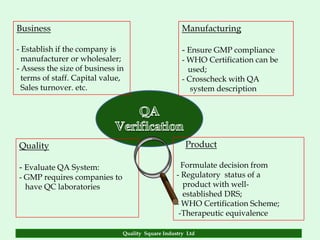
The document focuses on the importance of quality assurance (QA) in the pharmaceutical industry, emphasizing that QA encompasses all factors that affect product quality. It details processes and standards for ensuring quality from raw materials to finished products, highlighting the significance of compliance with good manufacturing practices (GMP) and ISO standards. Additionally, it outlines various quality improvement strategies, documentation protocols, and the role of technology in assuring product quality.