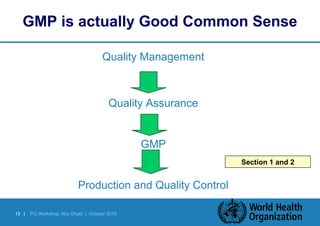
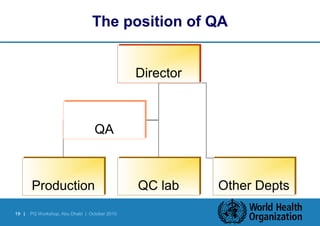
Good Manufacturing Practices (GMP) provide assurance that medicines are manufactured safely, effectively and with quality. GMP involves implementing quality management systems and following inspection guidelines to ensure compliance with standards during production. Inspections verify that manufacturing matches the authorized dossier and marketing approval. GMP guidelines cover APIs, finished pharmaceuticals, and other areas like personnel, facilities, equipment, materials, documentation, production, and quality control. Senior management plays a key role in continuously improving GMP implementation.