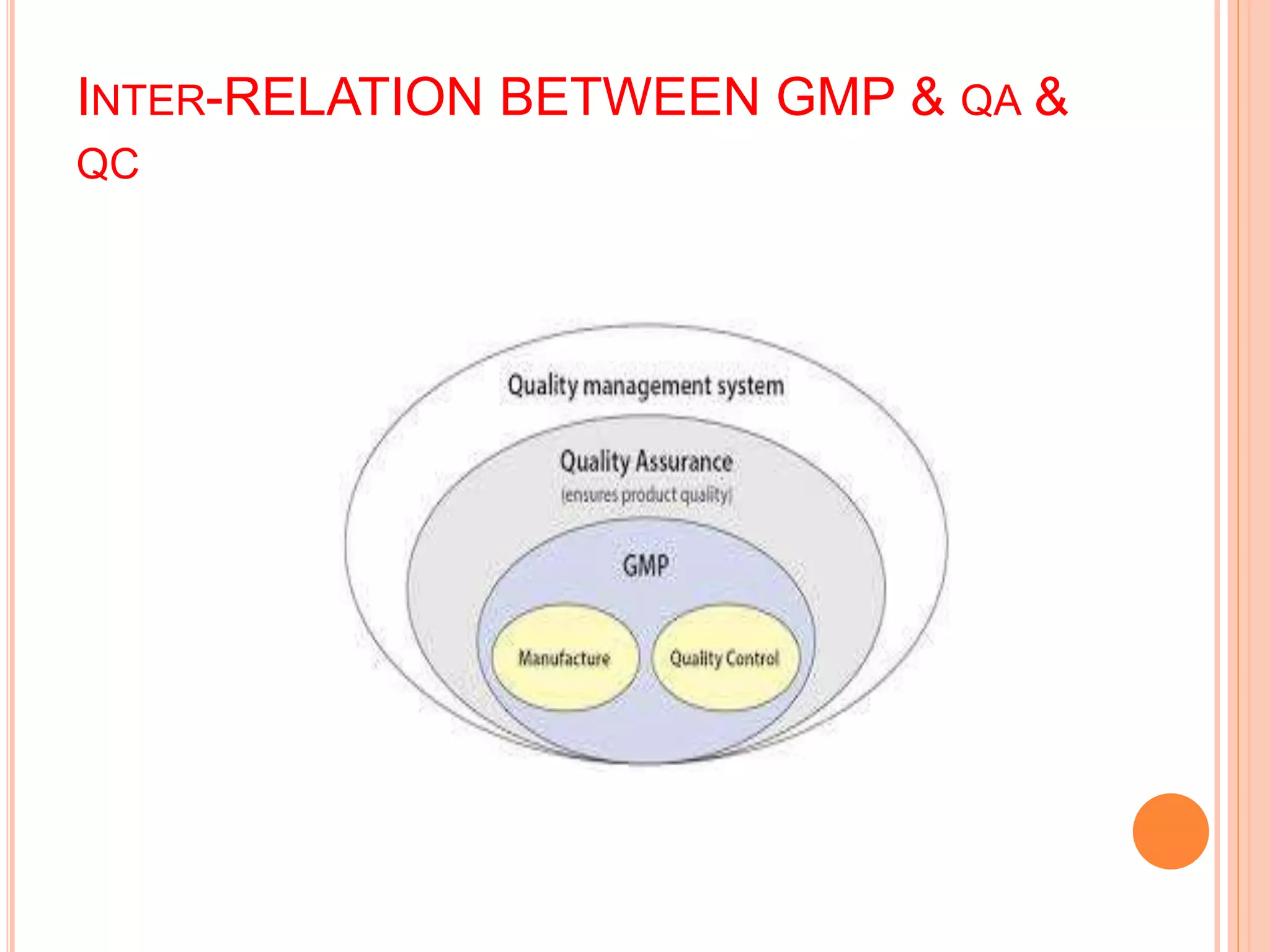
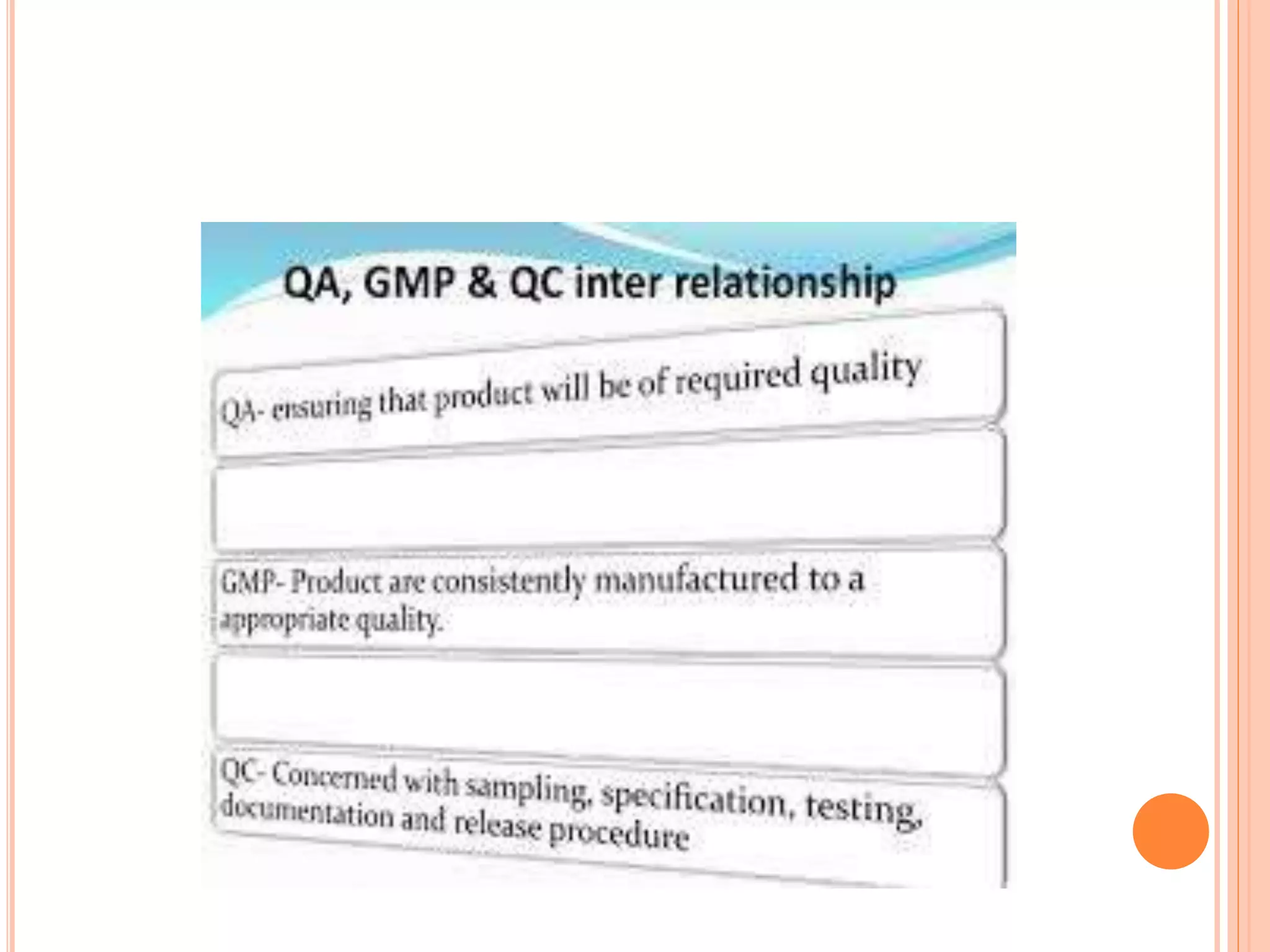
This document provides an overview of key concepts in pharmaceutical quality assurance and quality management, including the relationships between quality assurance (QA), quality control (QC), and good manufacturing practices (GMP). It defines QA, QC, and GMP and explains their roles and responsibilities. QA is a system that ensures products meet requirements, incorporating GMP and other factors. QC is the system for testing samples to maintain standards. GMP covers all production aspects to minimize risks and ensure quality, safety, and efficacy. Together, QA, QC and GMP work to consistently produce pharmaceuticals that meet quality standards.