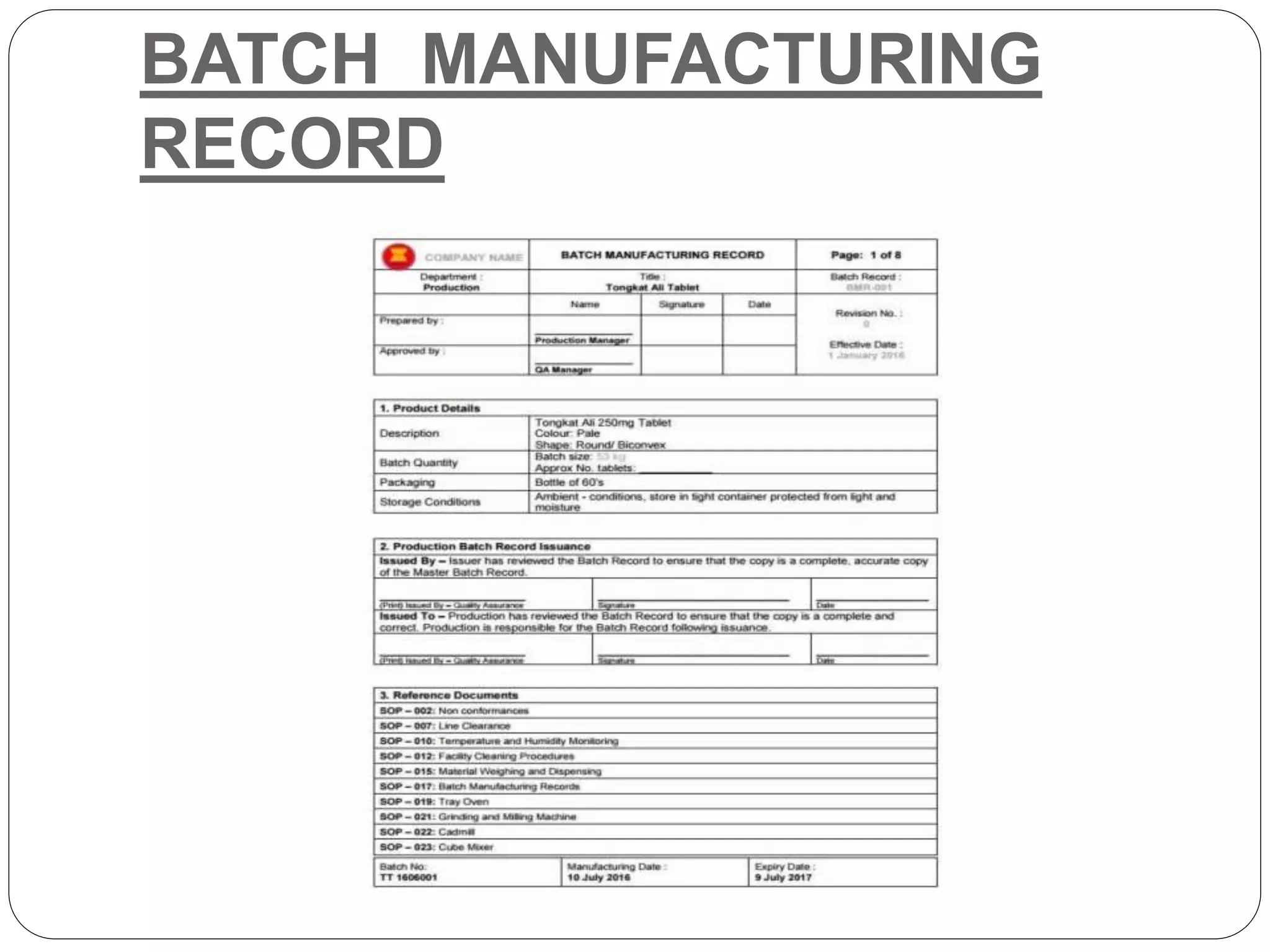
This document discusses batch manufacturing records (BMRs), which are necessary quality and GMP documentation used to trace the complete manufacturing cycle of a batch or lot of a product. A BMR contains information about the batch including the batch number, size, composition, manufacturing record, weight of drug, shelf life, and storage conditions. It also includes general manufacturing instructions, a cleaning record of equipment used, a bill of materials listing raw materials, step-by-step manufacturing process details, yield calculations, a list of abbreviations, and a history of changes made to the document. A good BMR format contains all of this essential information.




![BATCH MANUFACTURING
RECORD
The batch manufacturing record [BMR] is the
necessary quality and GMP documentation for
tracing the complete cycle of manufacturing of a
batch or lot.
BMR may be prepared in local language.
BMR is a written document from the batch that is
prepared during the p’ceutical manufacturing
process.
A good BMR format should contain following
part:
1. Batch record: First page of the BMR has all
records about the batch as batch no. ,batch size
,composition MFR, wt of drug shelf life, storage
condition ,mfg date ,expiry date date of starting](https://image.slidesharecdn.com/bmr0-200902085221/75/BATCH-MANUFACTURING-RECORD-6-2048.jpg)