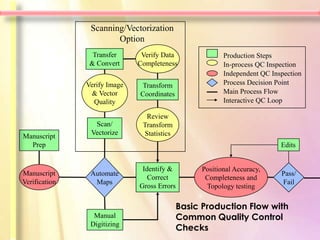
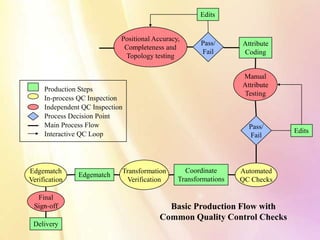
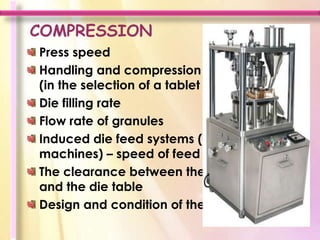
The document discusses in-process quality control for pharmaceutical manufacturing. It outlines that the purpose of in-process quality control is to ensure batch uniformity and integrity. It describes that procedures for in-process quality control should specify in-process controls and limits, required tests and examinations, and sampling procedures for each batch during both manufacturing and packaging operations. The document provides examples of key process parameters that should be evaluated and optimized as part of in-process quality control.