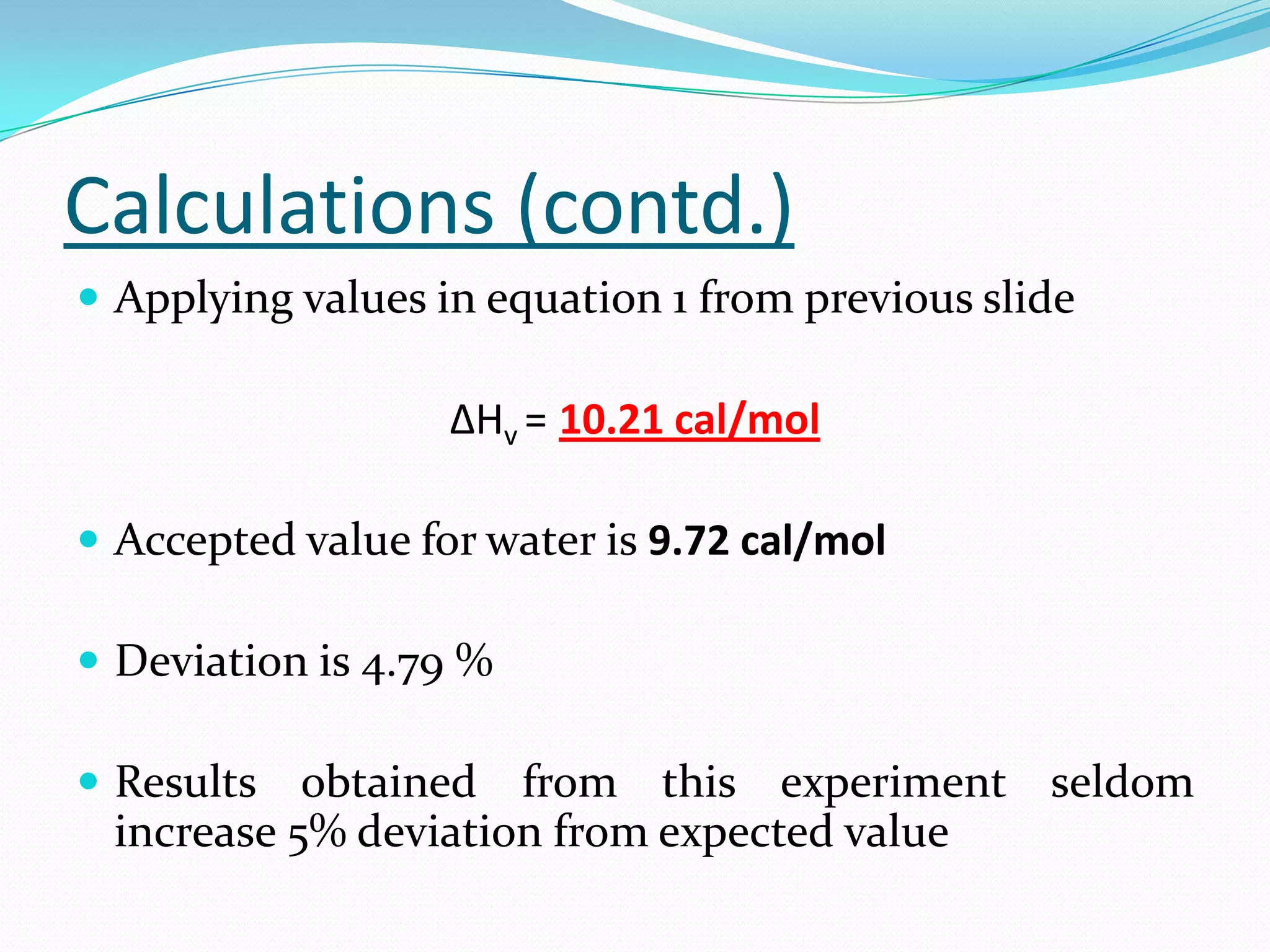
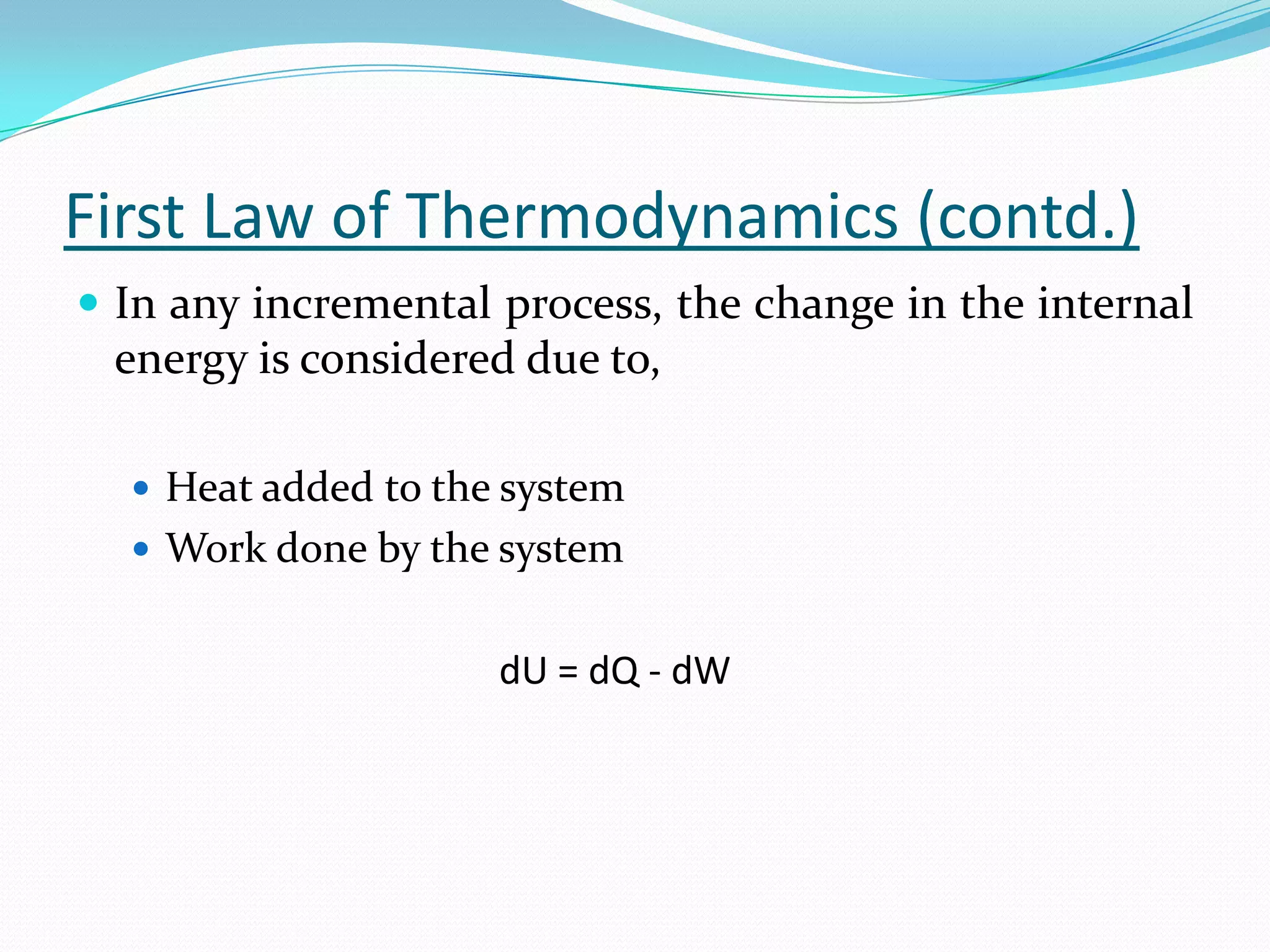
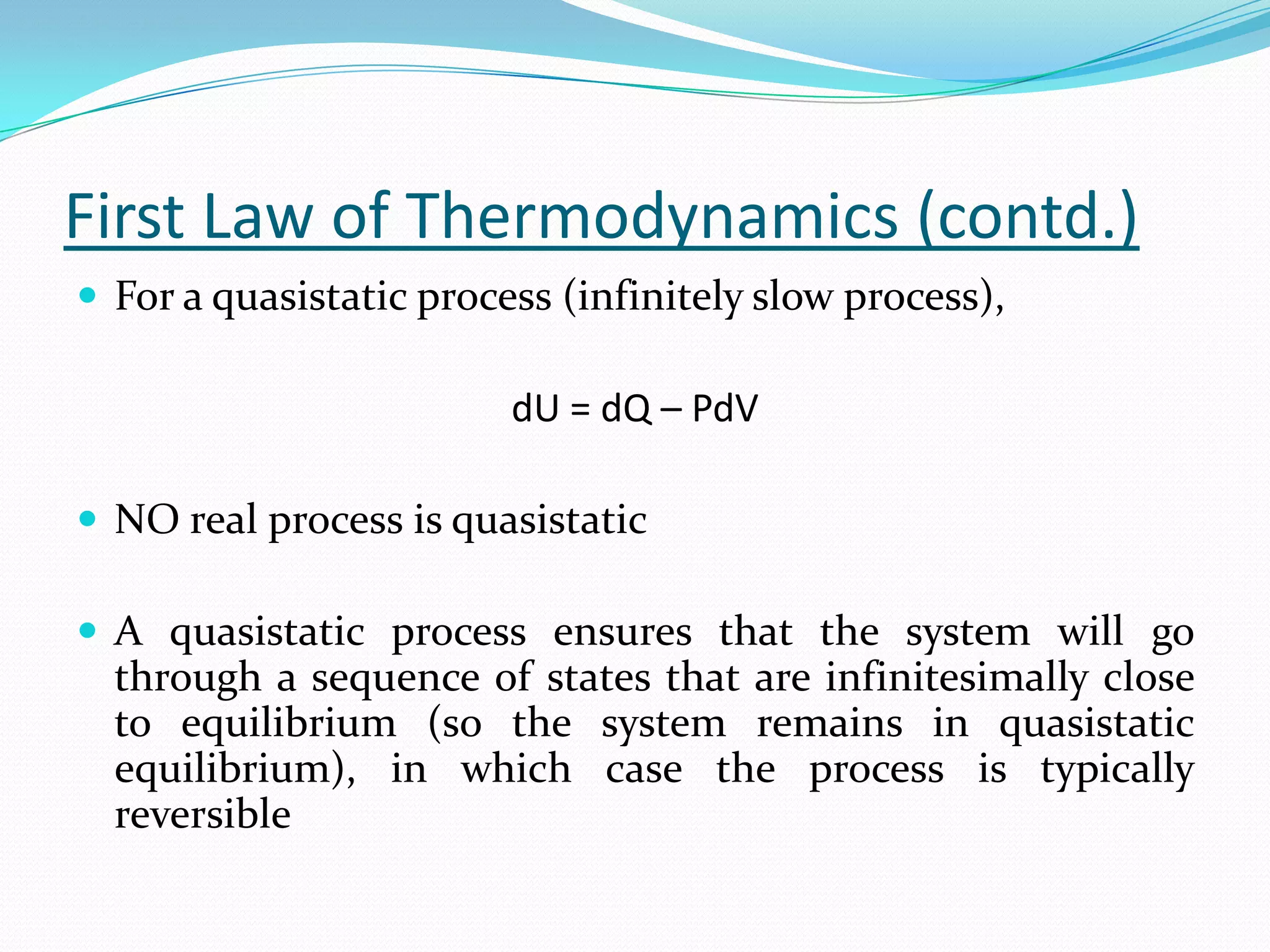
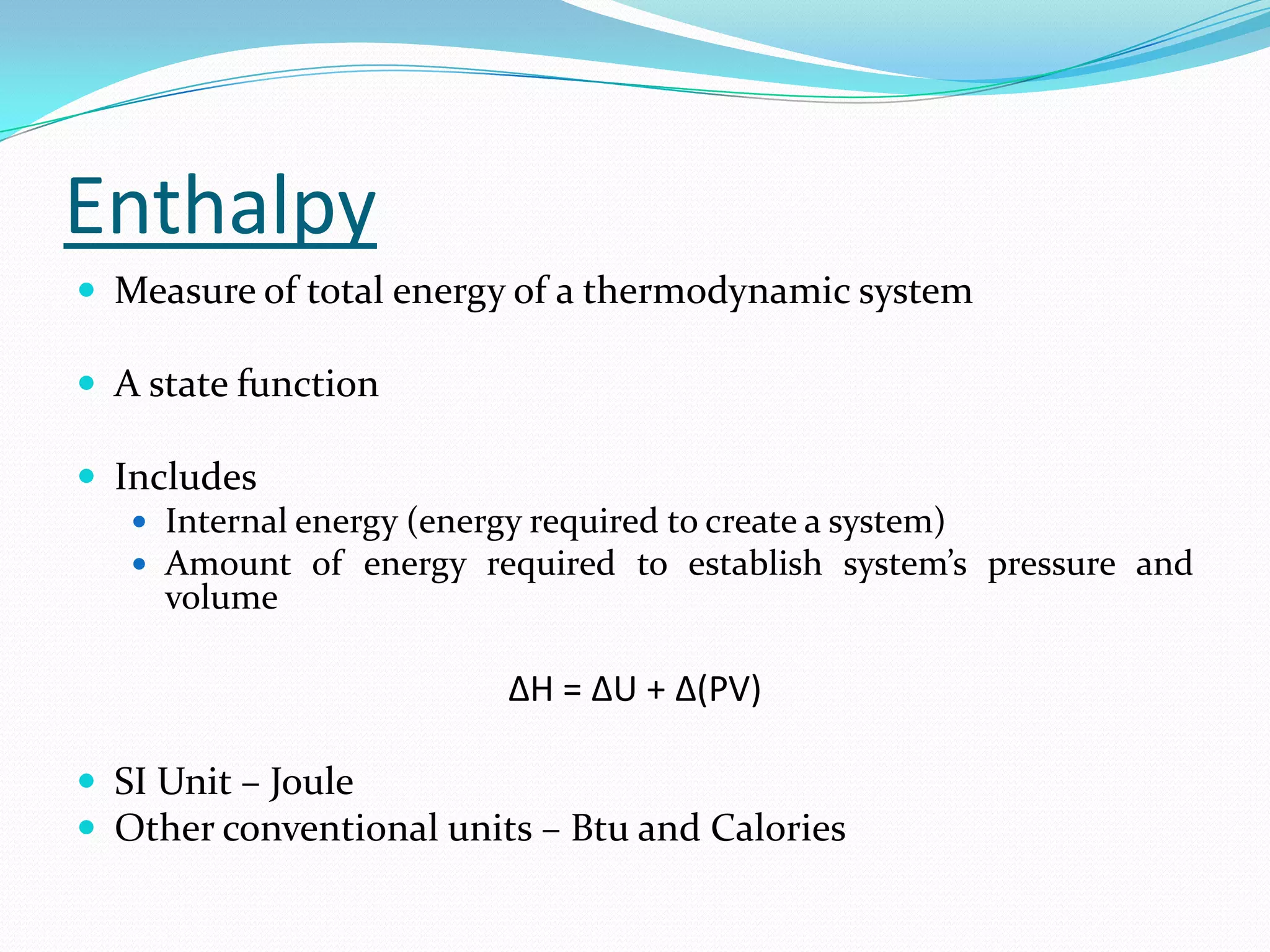
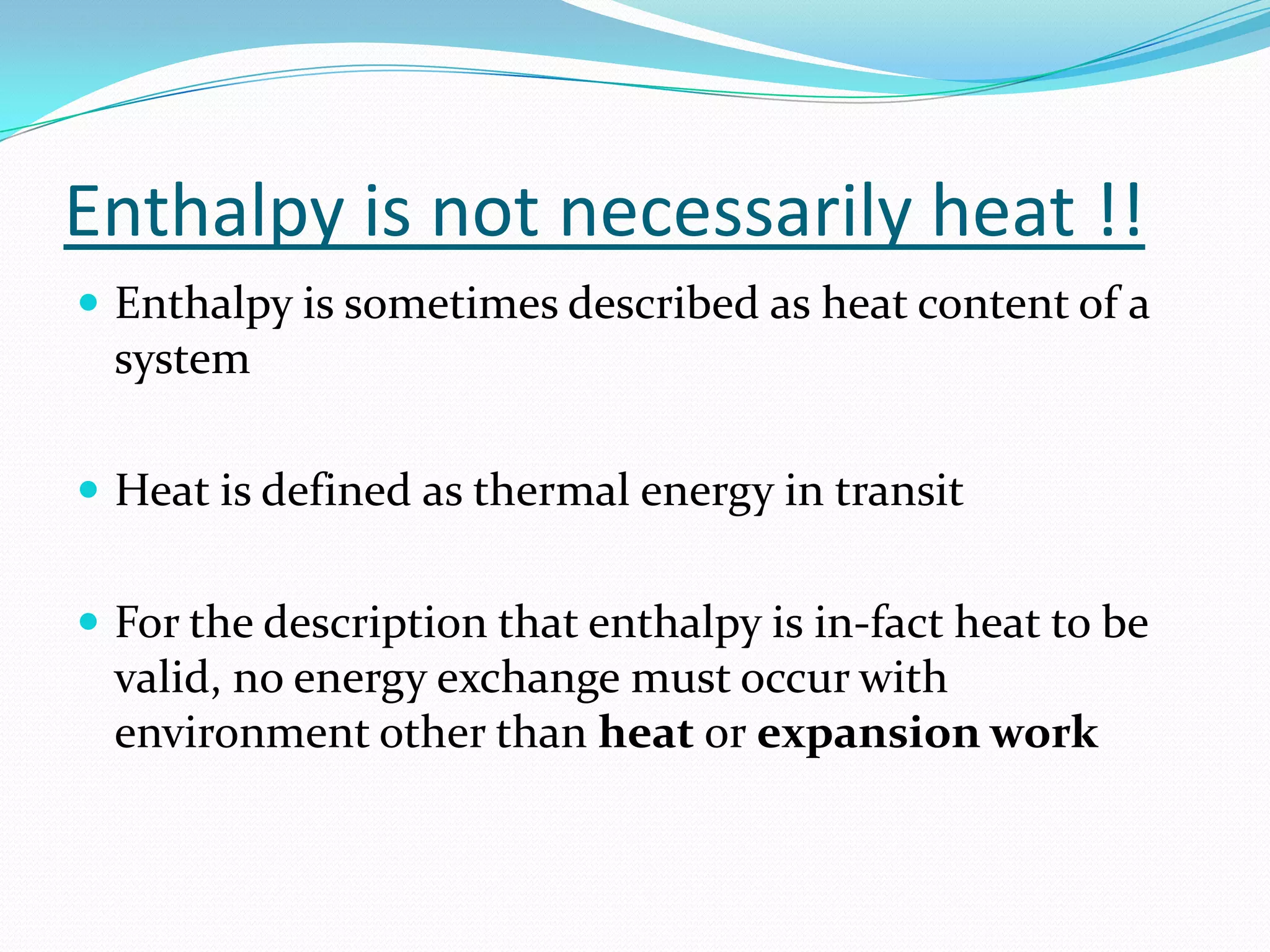
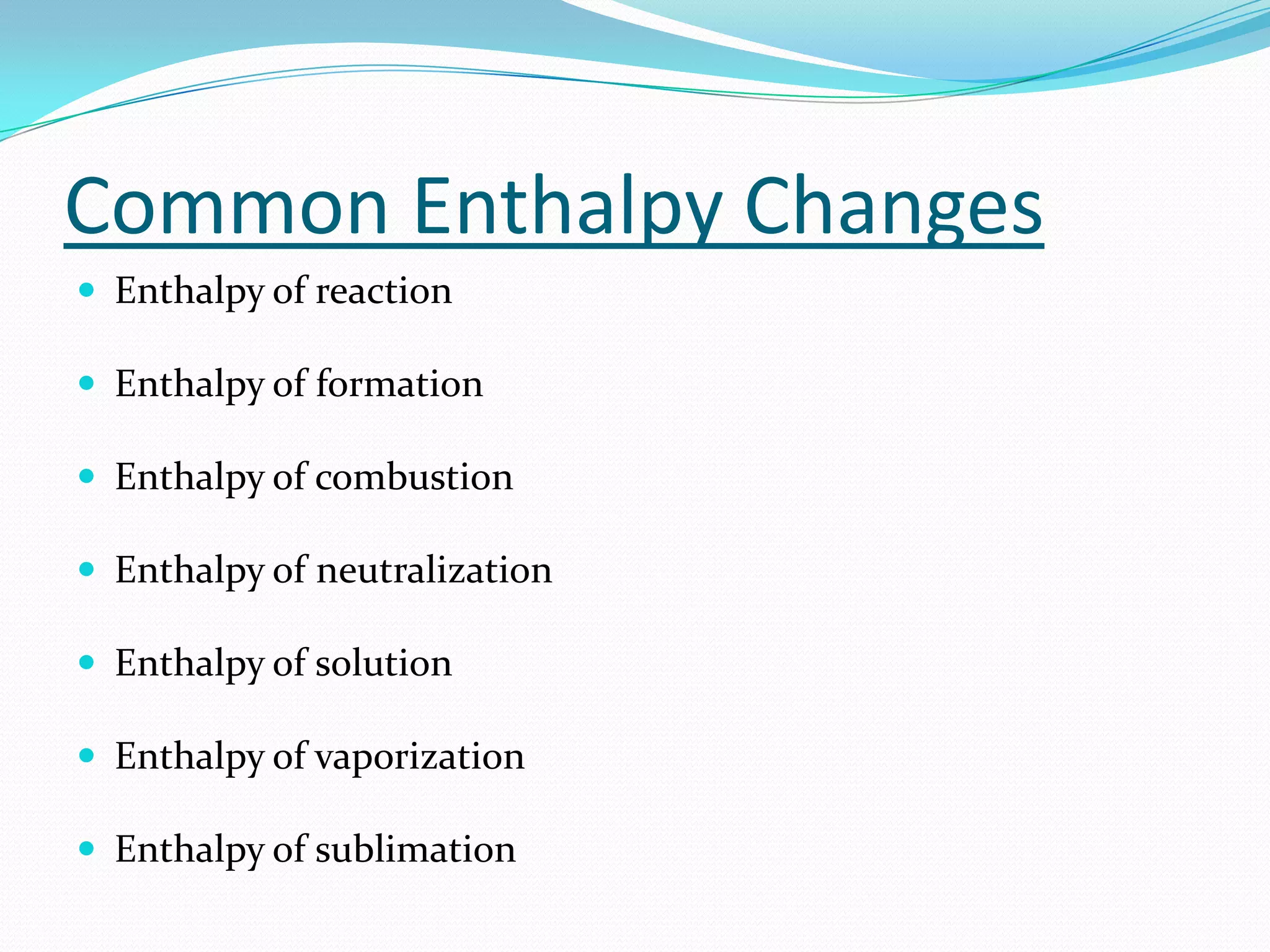
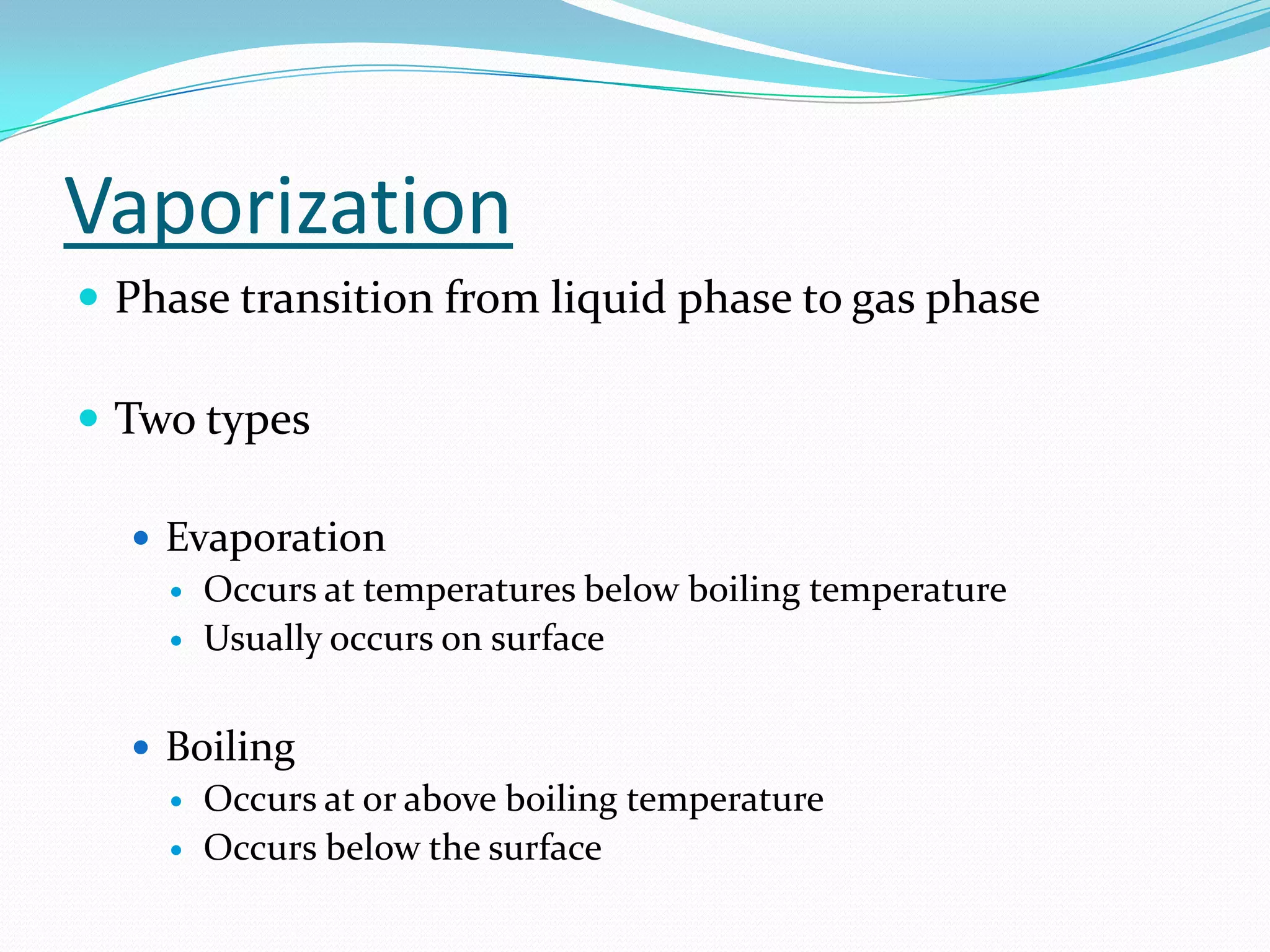
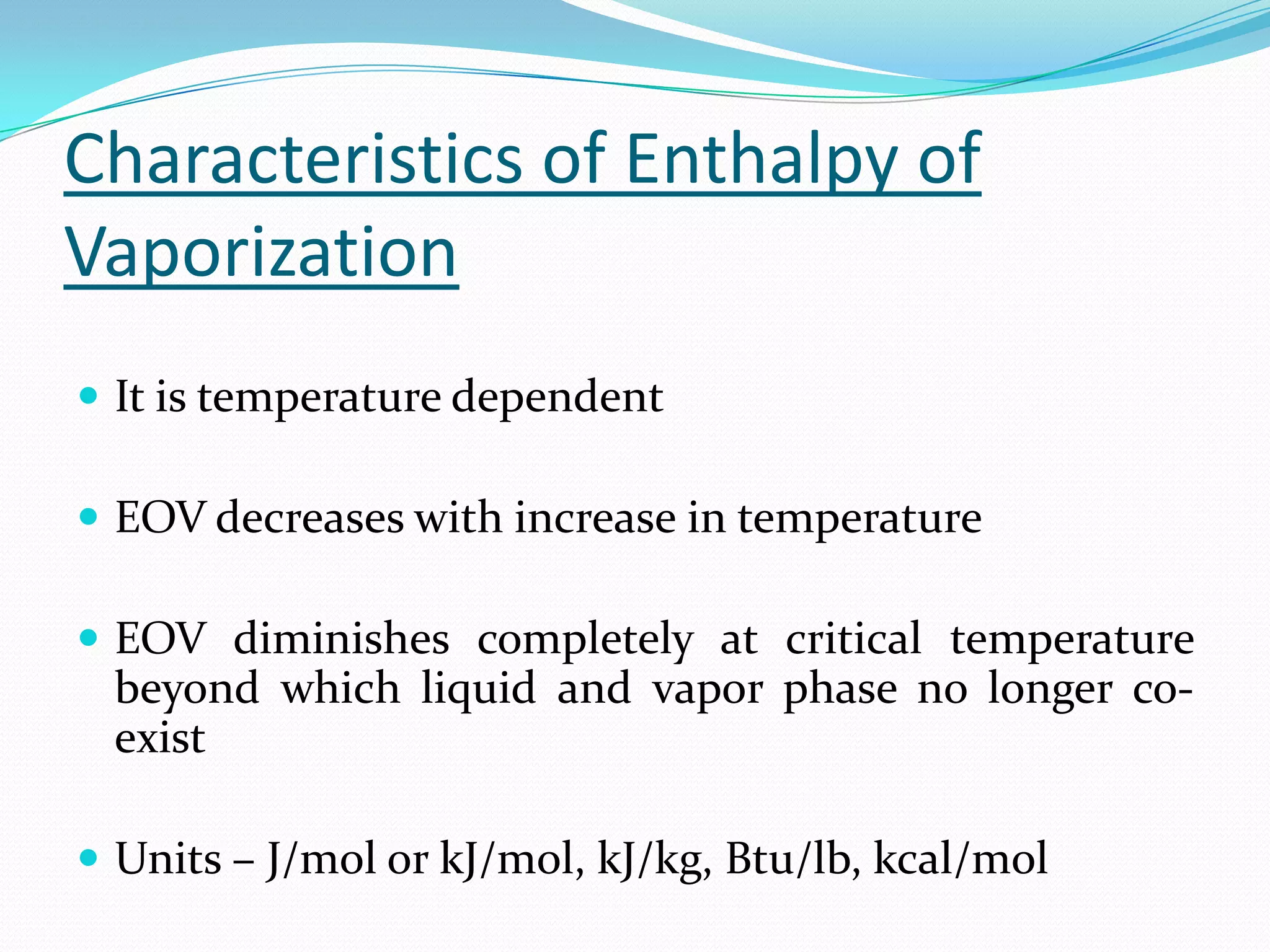
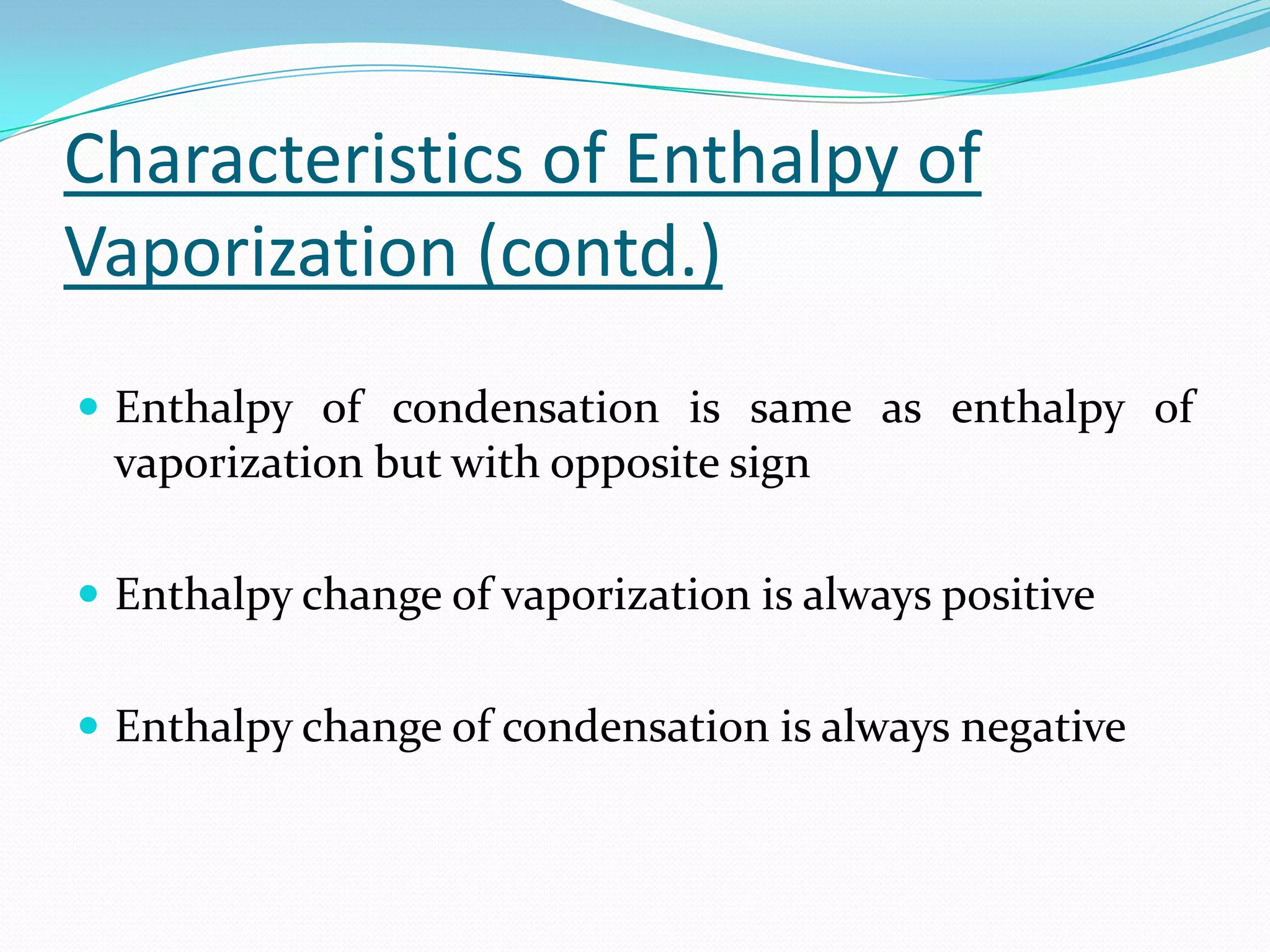
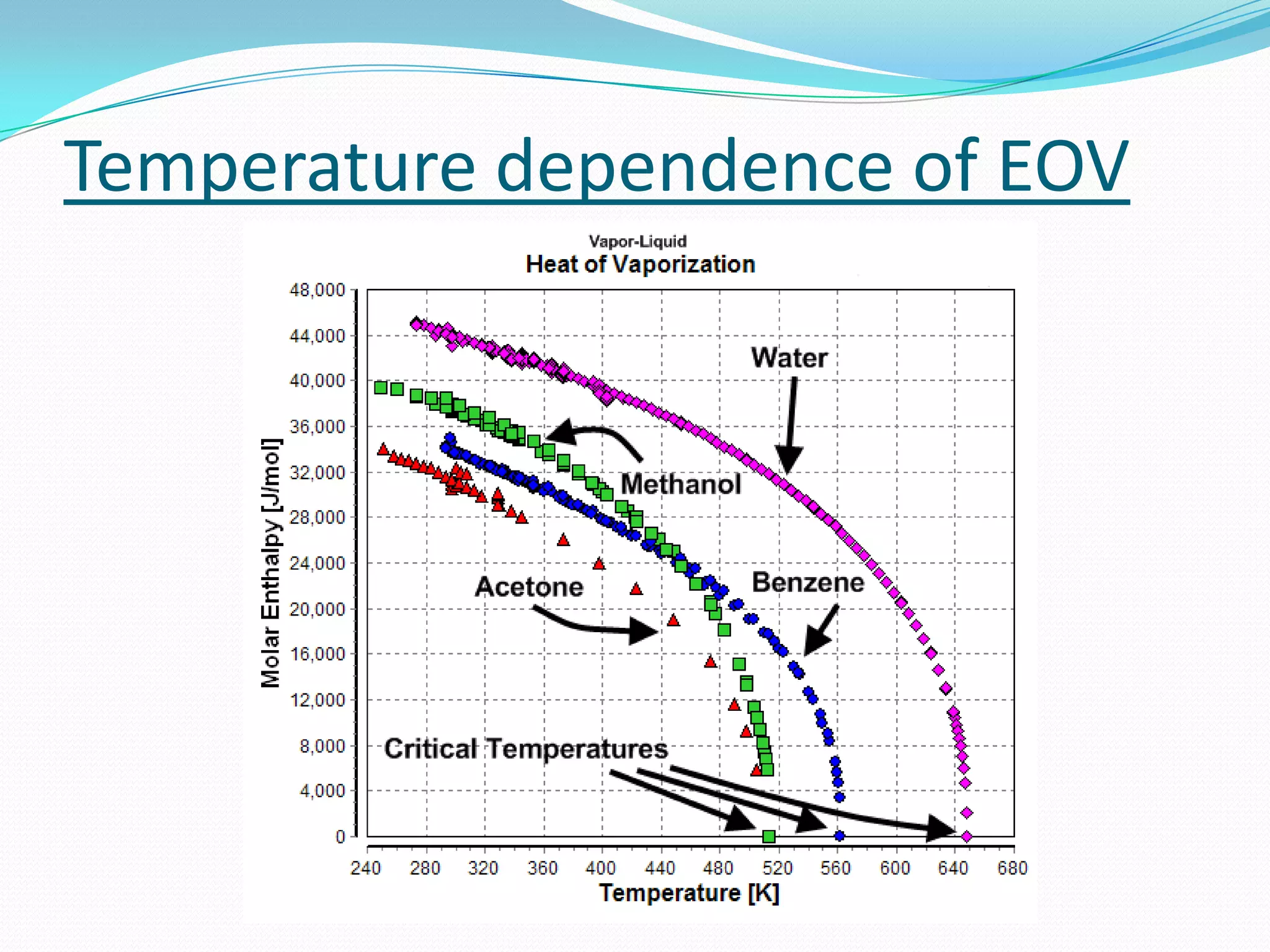
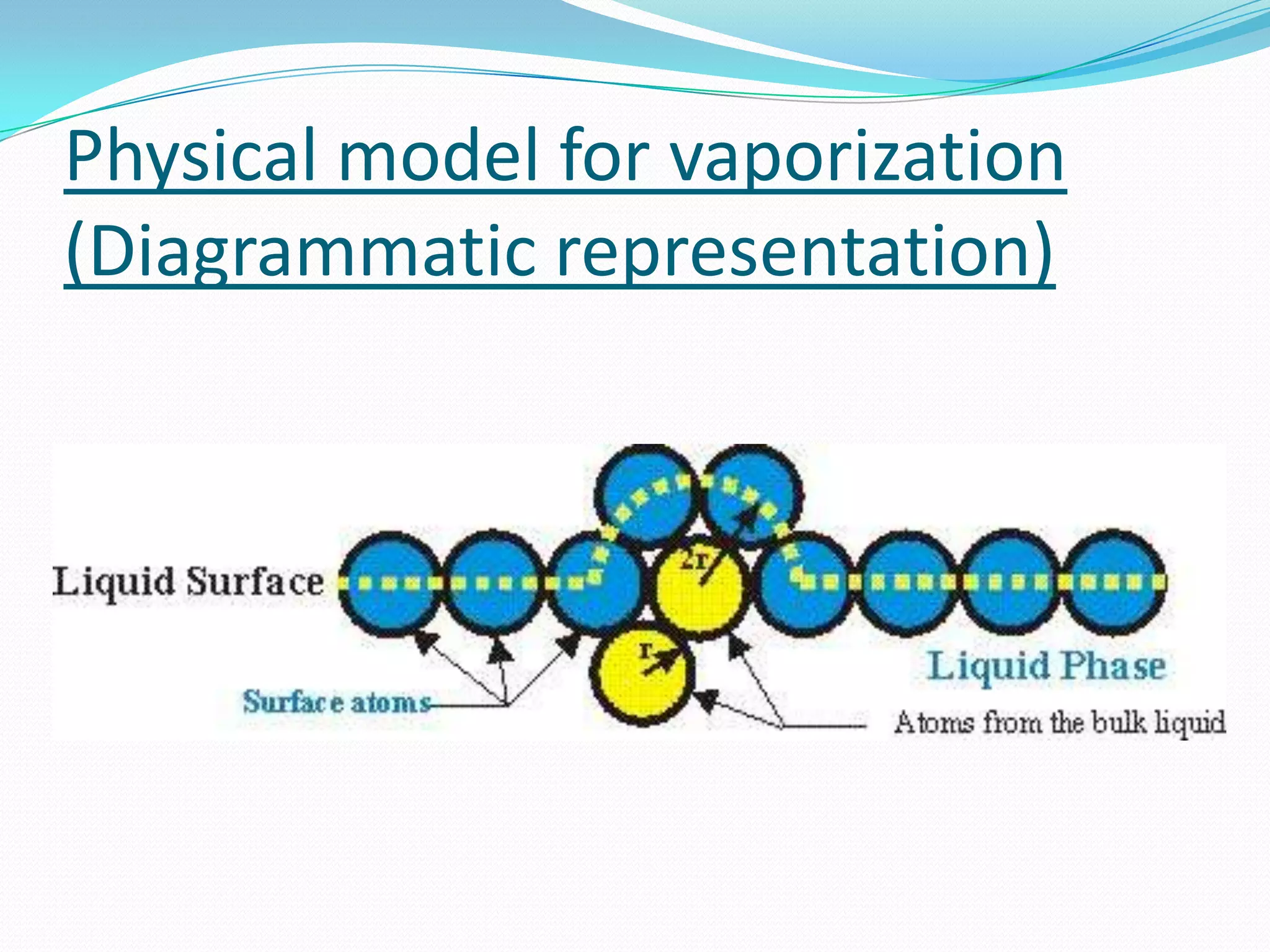
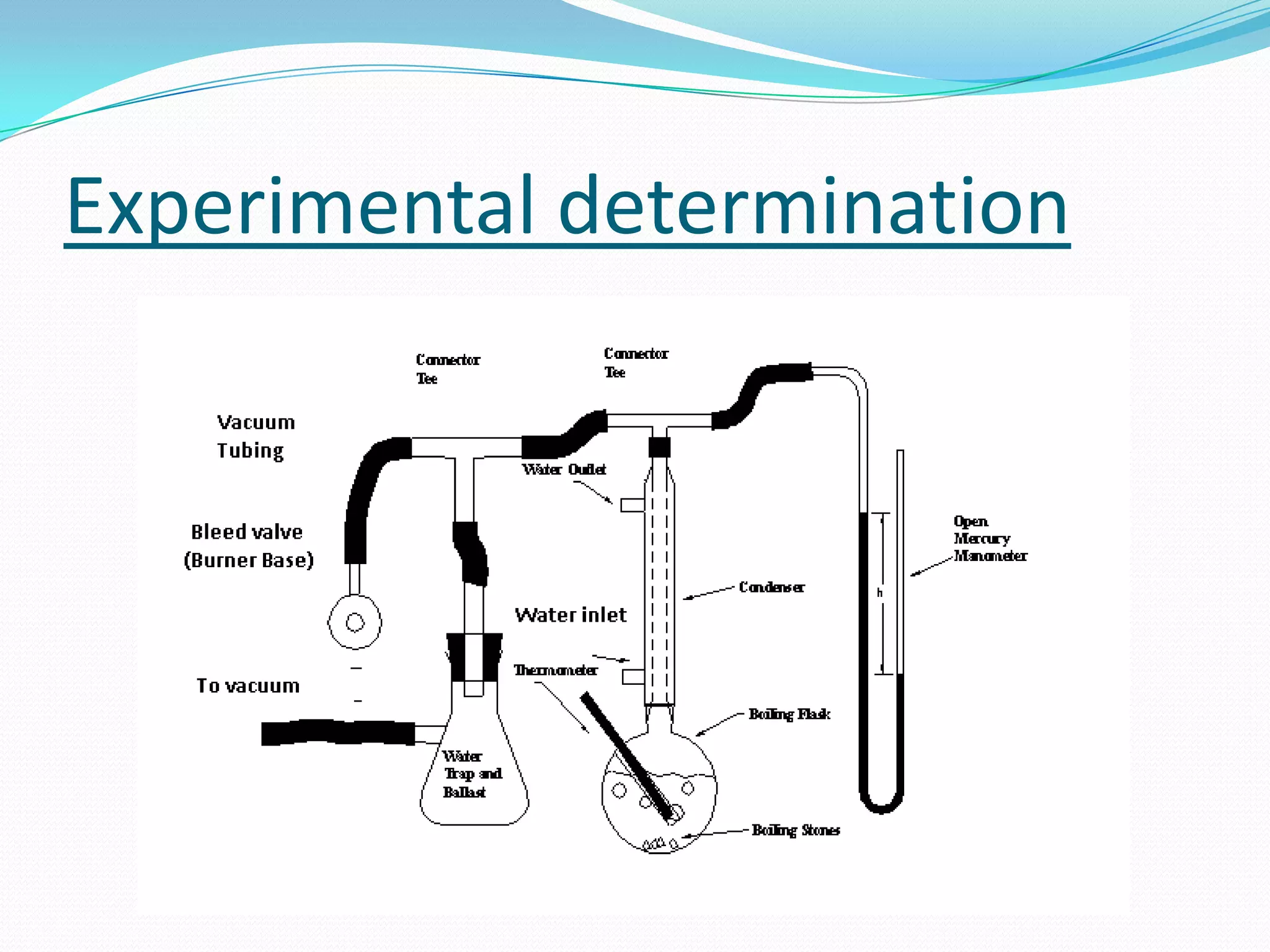
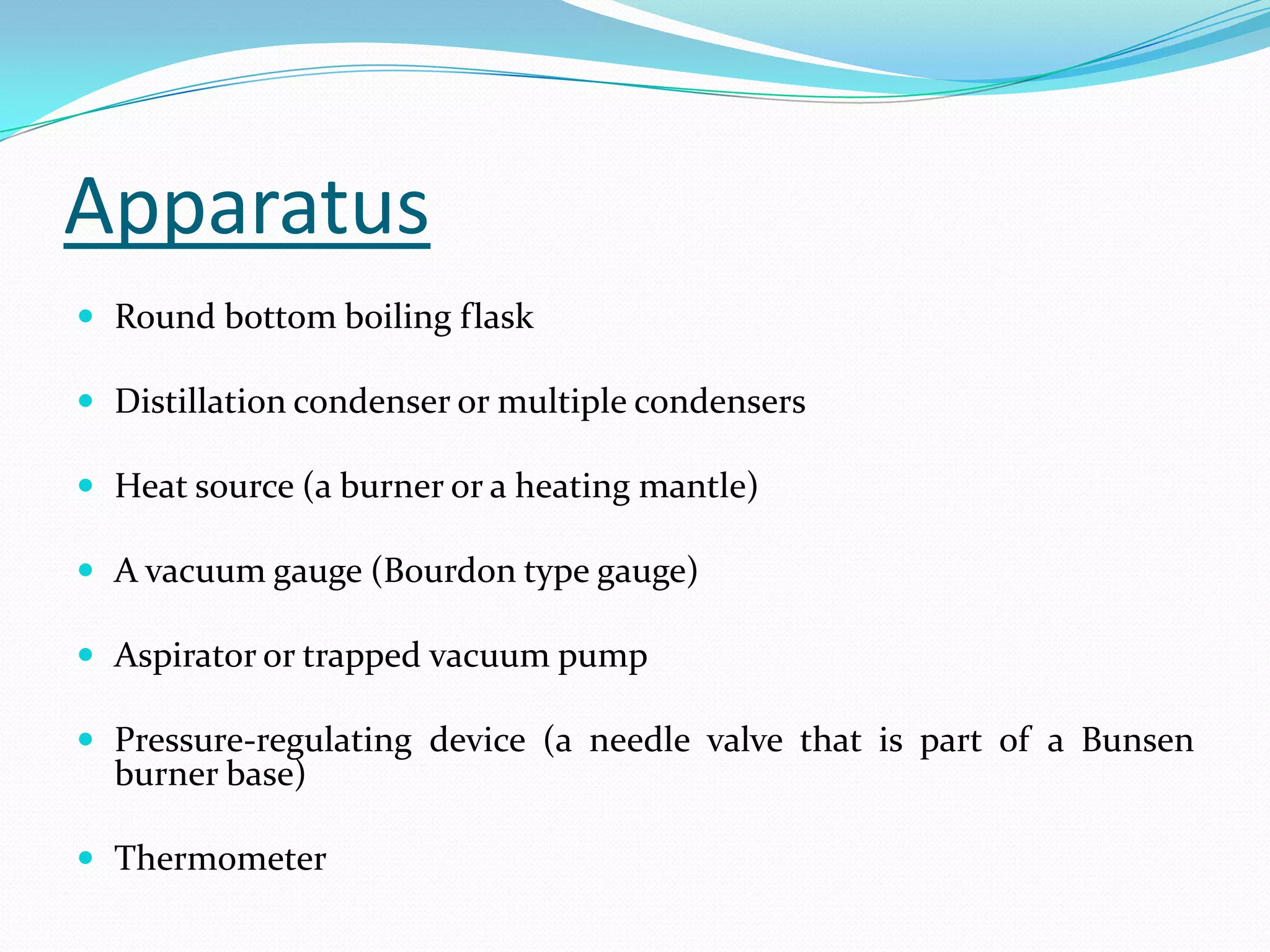
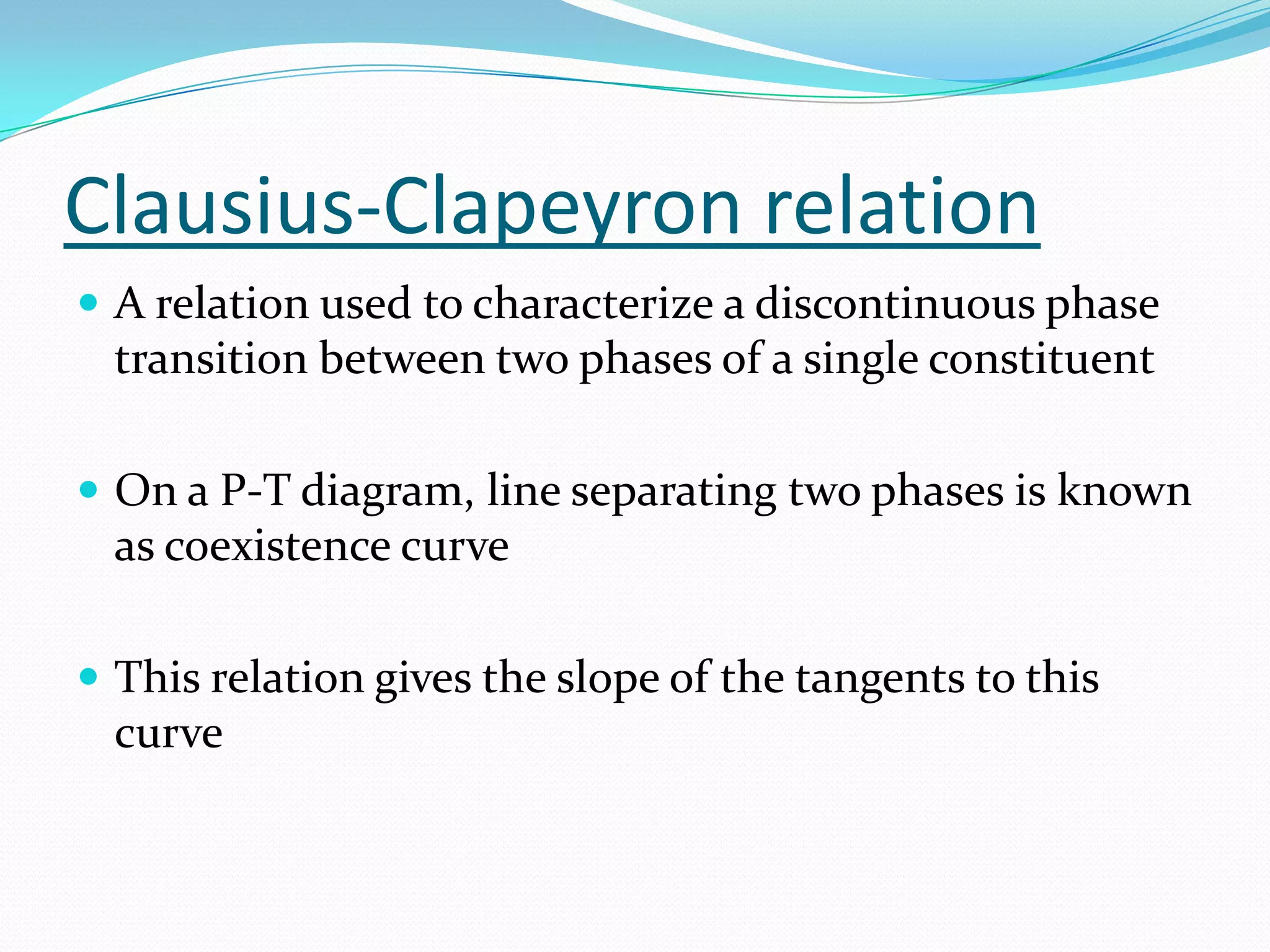
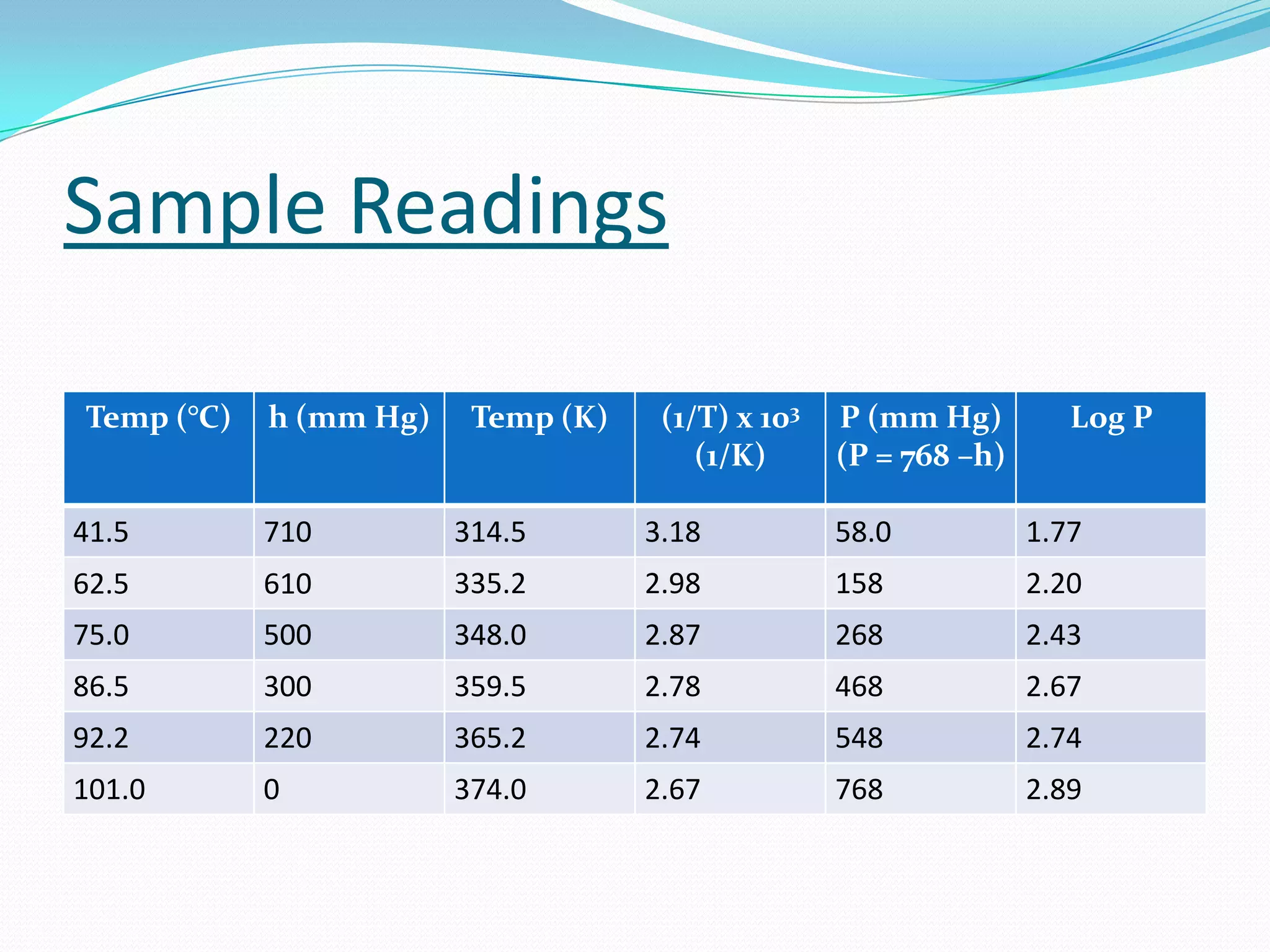
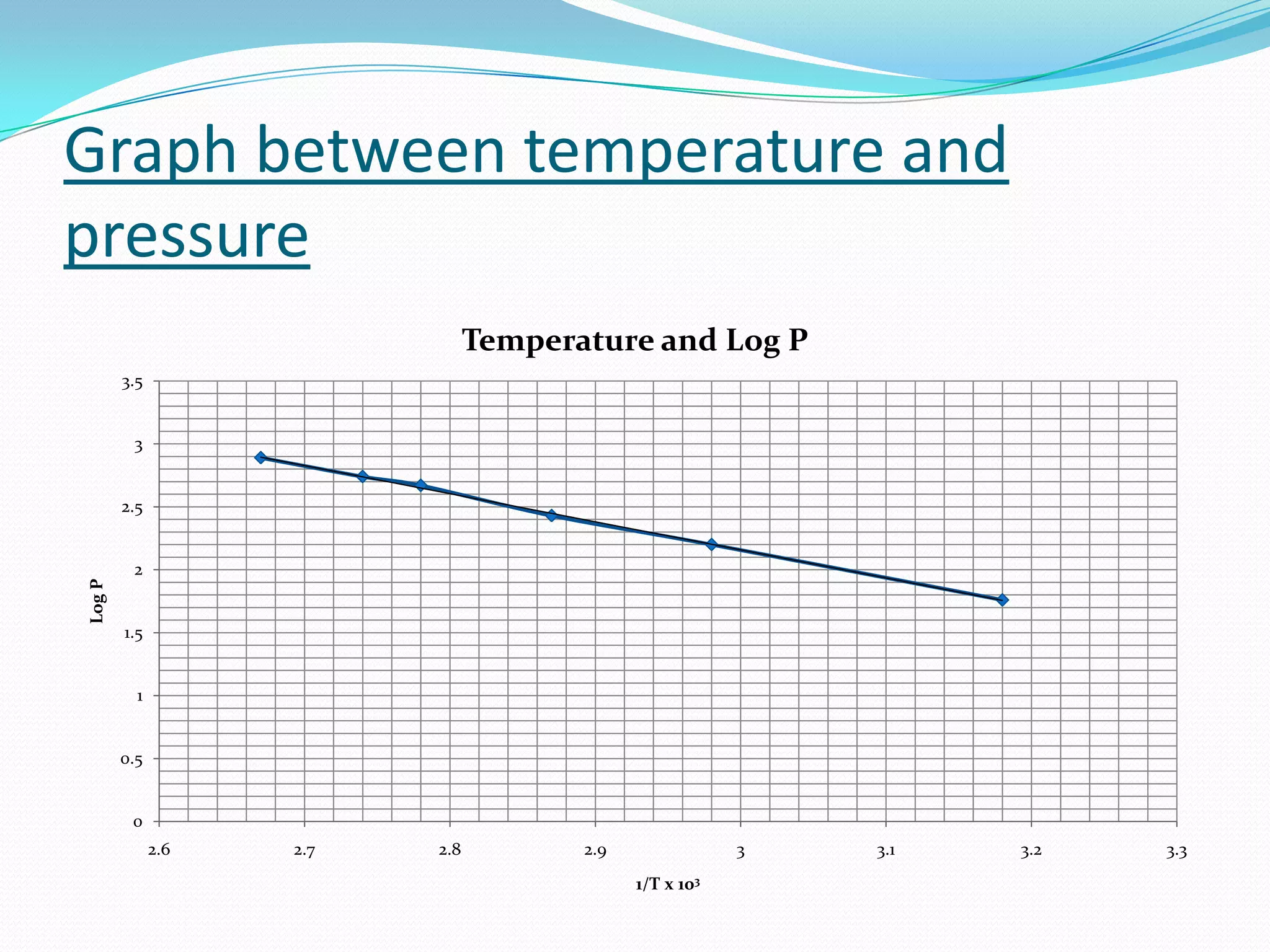
Seven students presented a detailed approach on thermodynamics. They restated the first law of thermodynamics and defined enthalpy. Common enthalpy changes were discussed, including enthalpy of vaporization. The characteristics and physical model of enthalpy of vaporization were described. An experiment was conducted to determine the enthalpy of vaporization of water at different pressures using a Clausius-Clapeyron analysis of temperature and pressure readings. The results were within 5% of accepted values. Applications of enthalpy of vaporization include steam power generation and distillation.







![Calculations
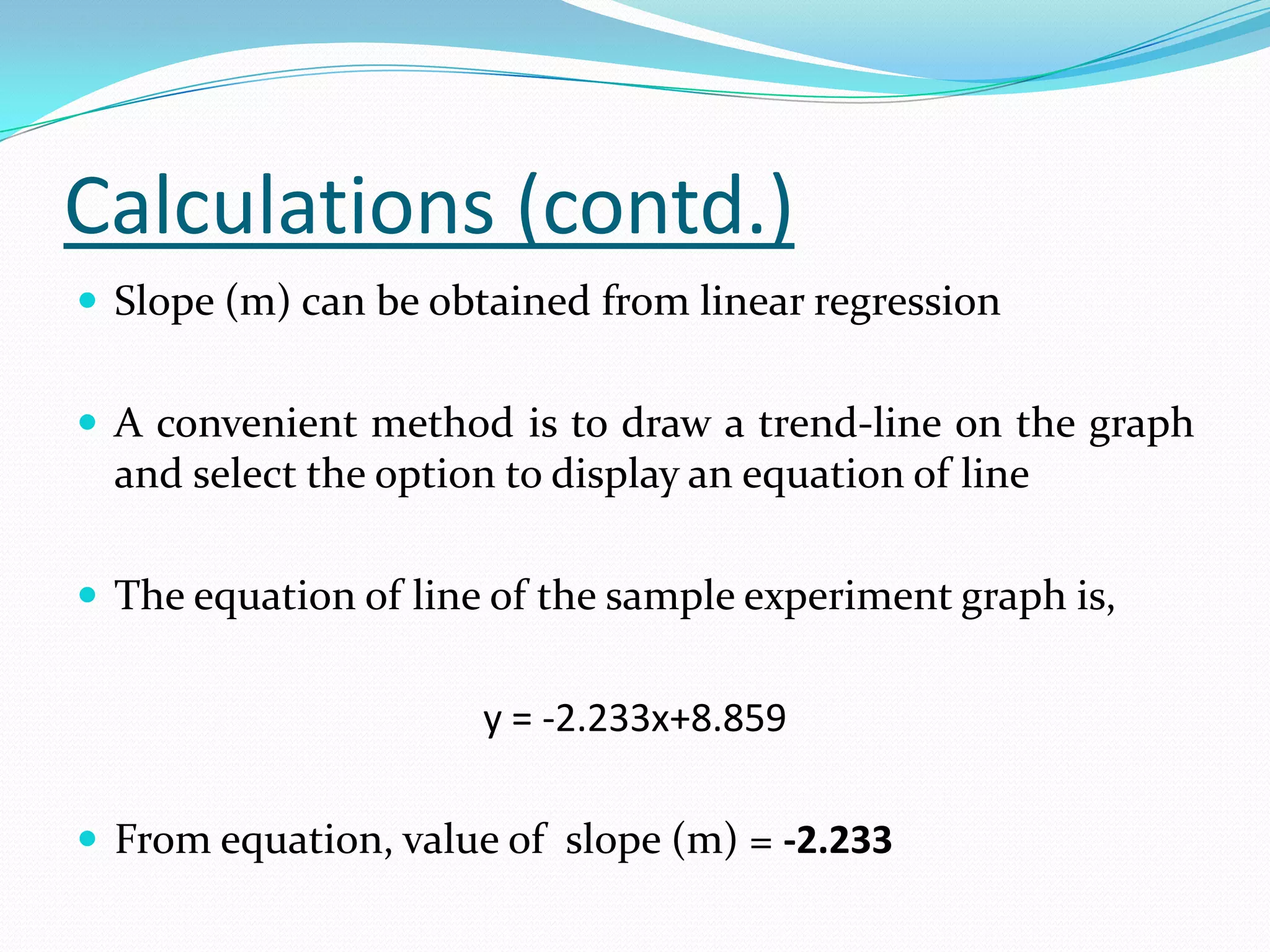
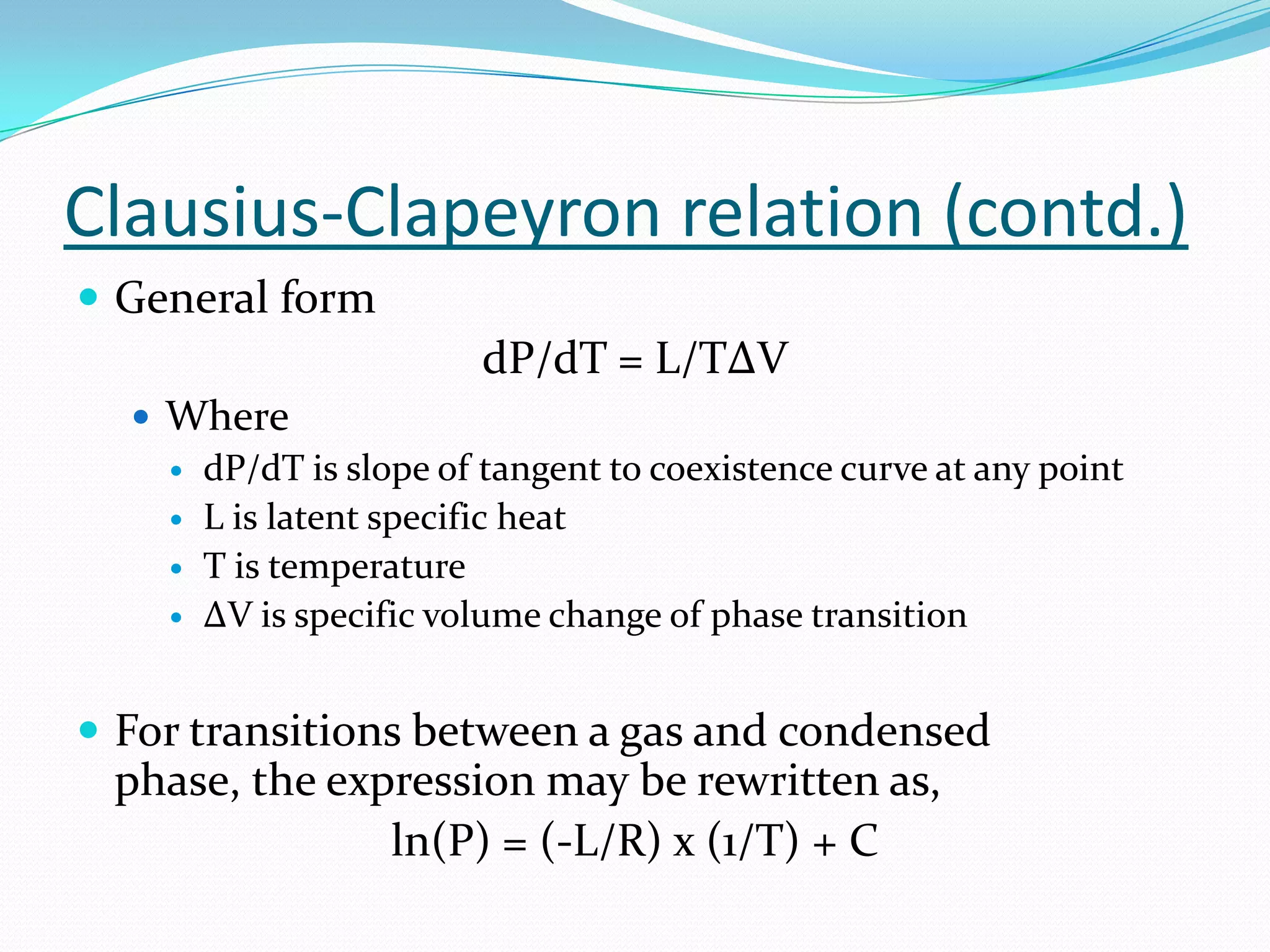
Molar latent heat / enthalpy of vaporization can be calculated
from Clausius-Clapeyron relation as follows,
ΔHv = -Rx[d(ln(P))/d(1/T)]]
ln(P) = 2.303 log(P)
Slope = m = d(ln(P))/d(1/T)
ΔHv = -2.303(R)(m) - - - - Eq. (1)
Where R is ideal gas constant = 1.987 cal/K
m is slope of line obtained from graph](https://image.slidesharecdn.com/enthalpyofvaporizationofliquid-120602062926-phpapp02/75/Enthalpy-of-vaporization-of-liquid-29-2048.jpg)