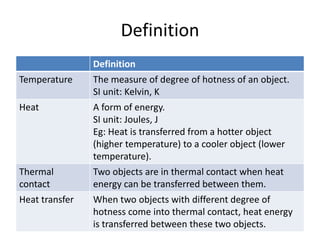
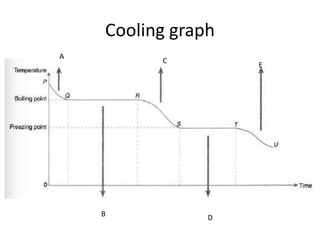
The document discusses heat and thermal equilibrium. It defines key terms like temperature, heat, and thermal contact. It explains that when two objects at different temperatures come into contact, heat is transferred from the hotter object to the cooler one until they reach the same temperature and thermal equilibrium. Examples are given like a wet towel being used to reduce a fever by transferring heat from the body. The document also discusses specific heat capacity and how it relates to how fast an object's temperature changes when heat is gained or lost. Specific heat capacities of different materials are provided.


























![Example 1:
• Calculate the total heat that is absorbed by a copper
block of mass 500g which has been heated from 31˚C
to 80˚C.
[Specific heat capacity of copper = 390 J kg-1 ˚C-1]](https://image.slidesharecdn.com/carmenphysicsheat-160713161011/85/SPM-Form-4-Physics-Heat-28-320.jpg)
![Example 2:
• When an electric heater is supplied with an electric
power of 2kW to heat 4kg of water for 1 minute,
calculate the increase in temperature of the water.
[specific heat capacity of water = 4200 J kg-1 ˚C-1]
Assume there is no heat loss to the surrounding.](https://image.slidesharecdn.com/carmenphysicsheat-160713161011/85/SPM-Form-4-Physics-Heat-29-320.jpg)


























![Example 2:
• An electric kettle contains 3kg of water. Calculate
the amount of heat required to boil away all the
water after the boiling point has been reached.
[Latent heat of vaporization of water = 2.26x106 J kg-1]](https://image.slidesharecdn.com/carmenphysicsheat-160713161011/85/SPM-Form-4-Physics-Heat-58-320.jpg)
![Example 3:
• What is the quantity of heat that is required to convert 4g of
ice into steam at 100˚C?
[specific latent heat of vaporization of water is 2.26x106 J kg-1 ;
specific latent heat of fusion of ice is 33600 J kg-1 ;
specific heat capacity of water = 4.2x103 Jkg-1 ˚C-1]](https://image.slidesharecdn.com/carmenphysicsheat-160713161011/85/SPM-Form-4-Physics-Heat-59-320.jpg)
















