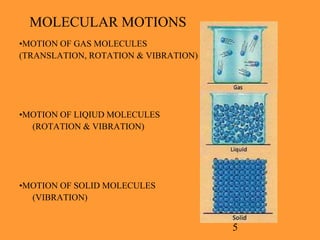
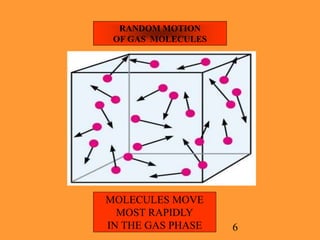
The document explains the Kinetic Molecular Theory (KMT), which describes the behavior of gases in terms of molecular energy states, motions, and interactions. It outlines the differences between ideal gases, which conform to KMT postulates, and real gases, which exhibit molecular volume and attractive forces, leading to deviations from ideal gas behavior. Understanding KMT enhances comprehension of gas laws and general chemistry principles.