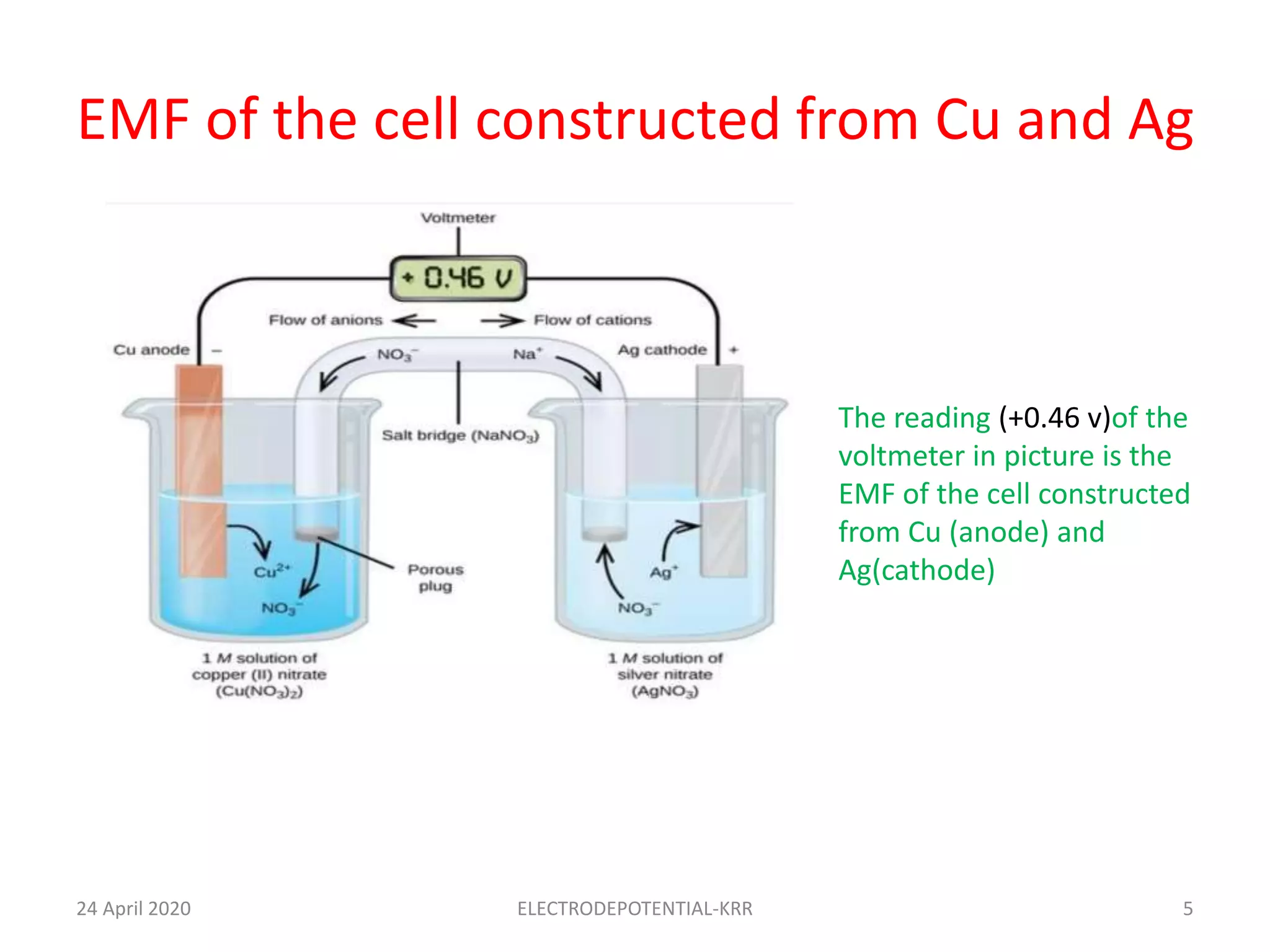
This document discusses the electromotive force (EMF) of galvanic cells. It defines EMF as the potential difference between electrodes that causes electron flow. The EMF of a cell is calculated as the cathode reduction potential minus the anode reduction potential. As an example, the EMF of a cell constructed from a copper anode and silver cathode is +0.46 V. The document also provides four numerical problems calculating EMF values for different metal combinations using their standard reduction potentials.