Embed presentation
Downloaded 16 times
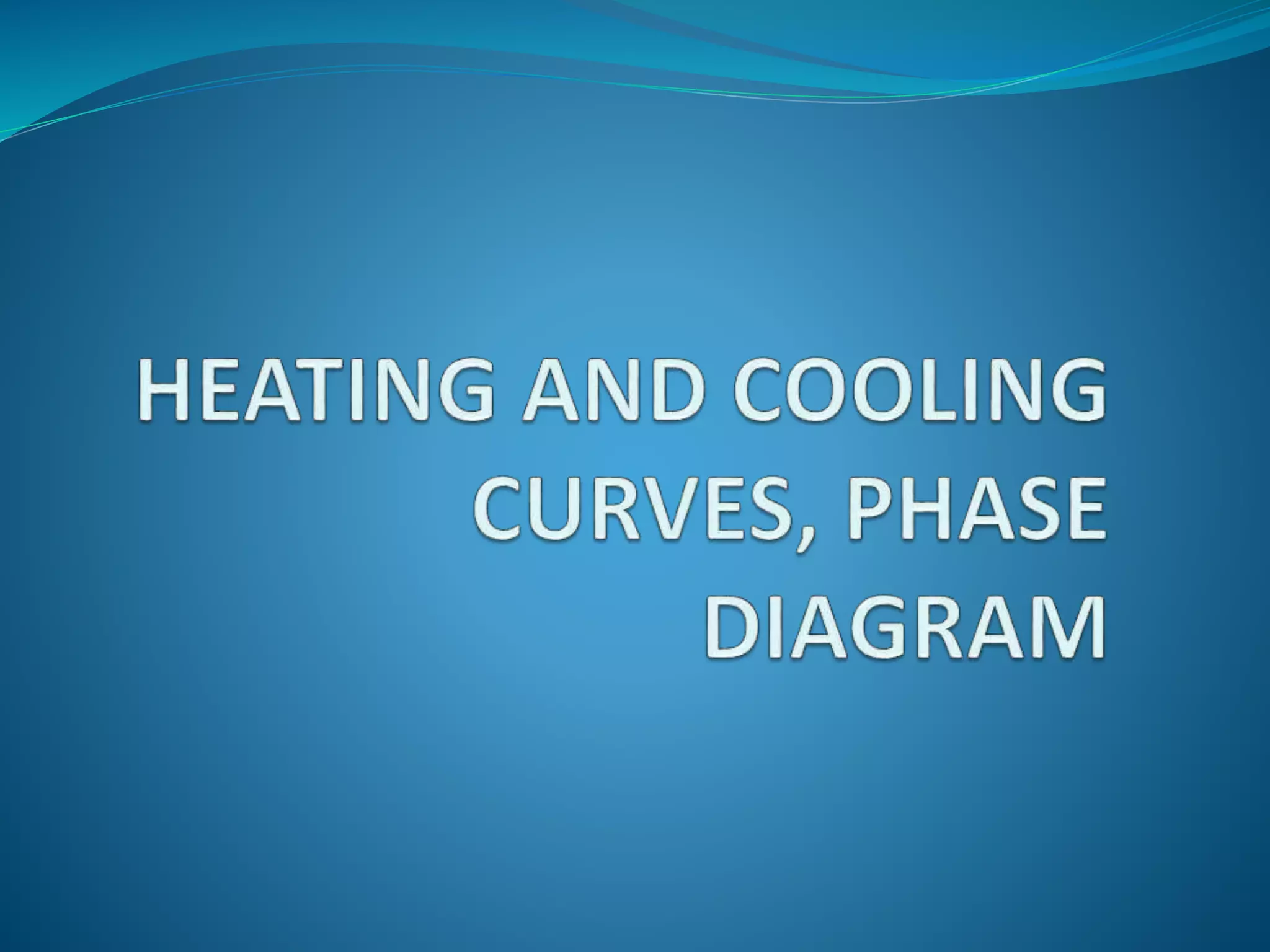
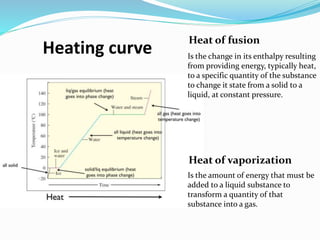
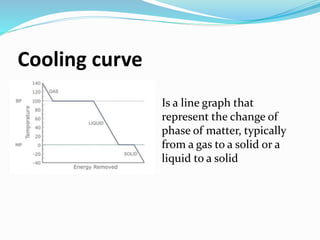
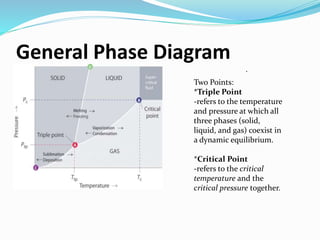

The document discusses key concepts related to phase changes of matter including heat of fusion which is the energy required to change a substance from a solid to a liquid, heat of vaporization which is the energy required to change a liquid to a gas, cooling curves which show phase changes from gas to solid or liquid to solid, and phase diagrams which illustrate phase boundaries including the triple point where solid, liquid, and gas phases coexist and the critical point which is the highest temperature and pressure point of a substance in its gas phase.



