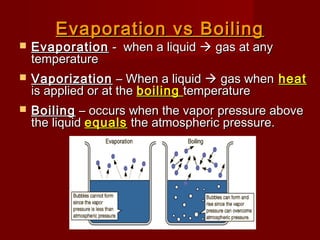
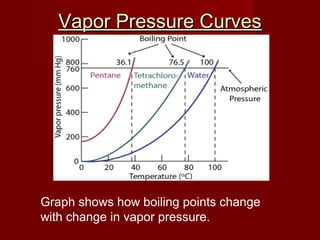
The document discusses vapor pressure and boiling points. It explains that vapor pressure increases with temperature as more liquid molecules escape into the gas phase, creating pressure above the liquid. Boiling occurs when vapor pressure equals atmospheric pressure. Boiling point depends on the strength of molecular attractions, with stronger attractions yielding higher boiling points. Boiling points change with pressure, with lower pressure resulting in lower boiling points and higher pressure yielding higher boiling points.