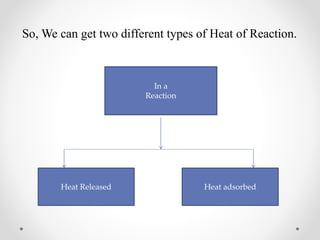
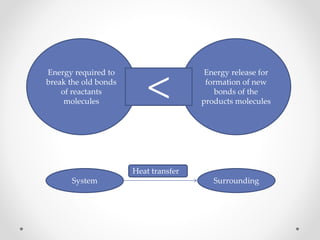
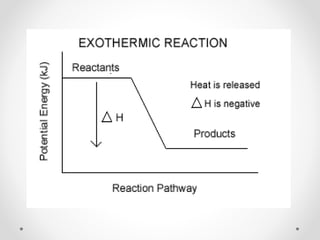
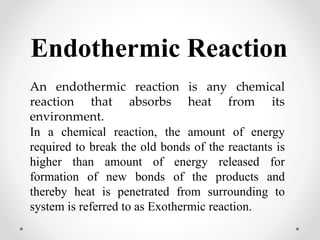
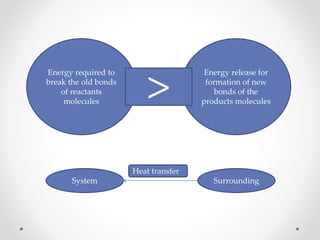
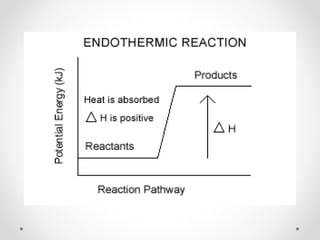
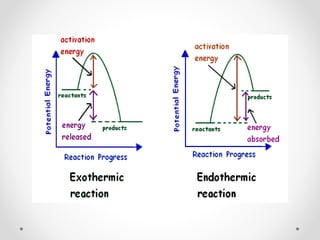
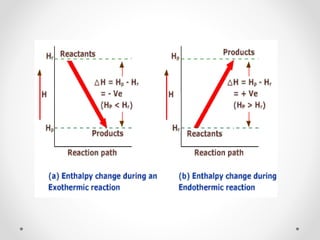
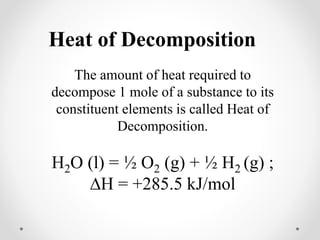
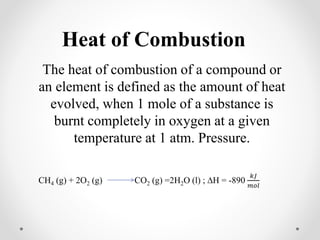
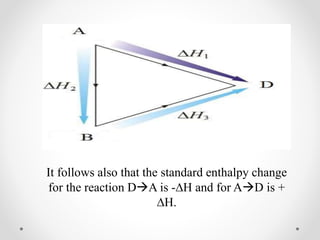
The document discusses thermochemistry, which is the study of energy and heat in chemical reactions and physical transformations, emphasizing concepts like exothermic and endothermic reactions. It outlines different types of heat reactions, including heat of formation, decomposition, combustion, and neutralization, along with Hess’s law, stating that total enthalpy change remains consistent regardless of the reaction path. Graphical representations illustrate energy changes for these reactions, detailing how energy is absorbed or released during chemical processes.