This document provides an overview of key concepts relating to gases, including:
- Characteristics of gases such as expanding to fill their container and being highly compressible.
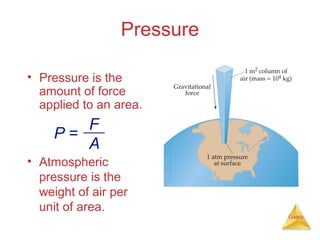
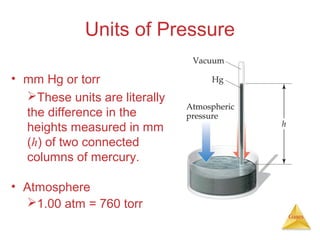
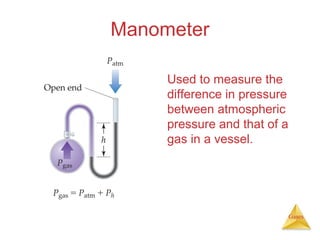
- Definitions and units used to measure gas pressure, such as pascals, bars, mmHg, and atmospheres.
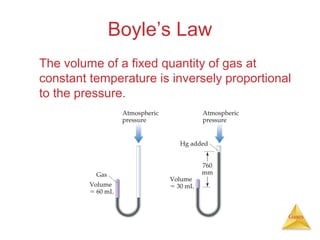
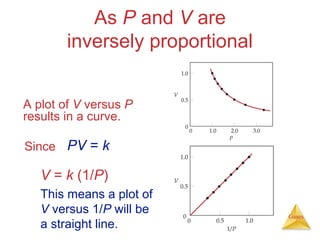
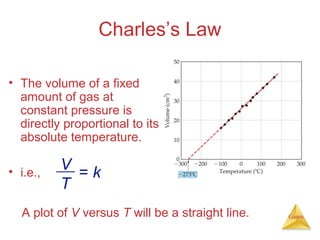
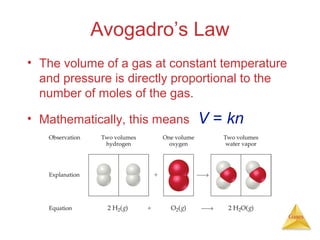
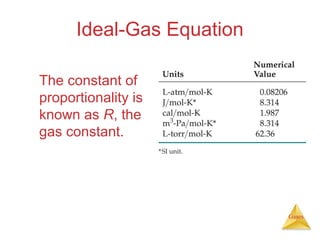
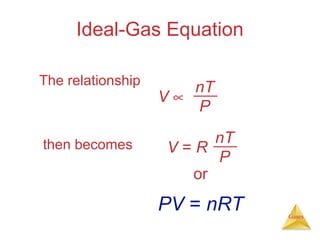
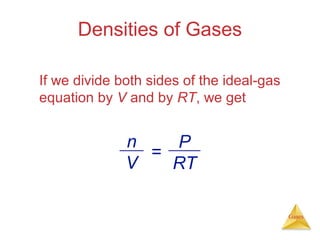
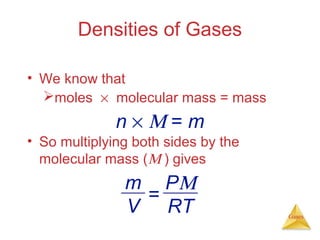
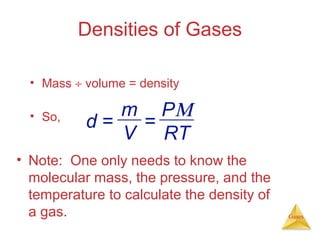
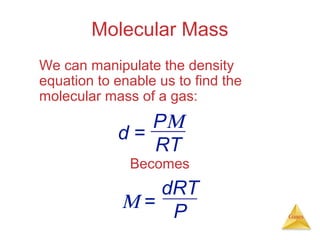
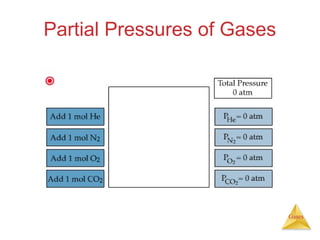
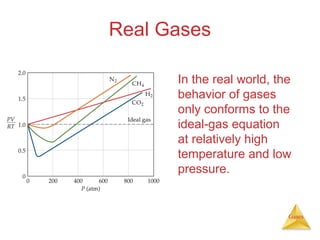
- Laws describing the behavior of gases, including Boyle's law, Charles's law, Avogadro's law, Dalton's law of partial pressures, and the ideal gas equation.
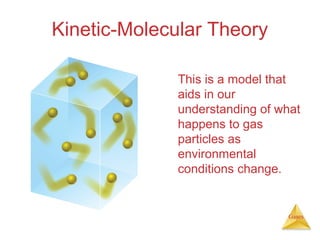
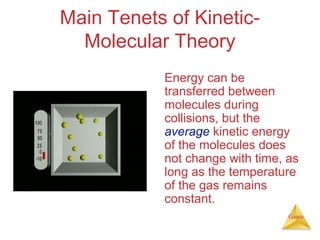
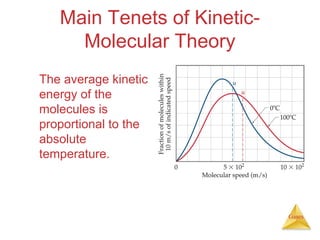
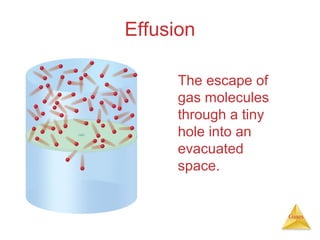
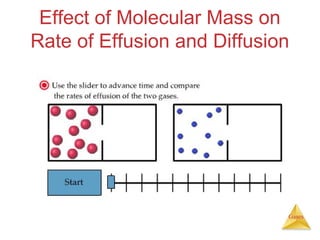
- The kinetic molecular theory which models the behavior of gas particles at the molecular level.