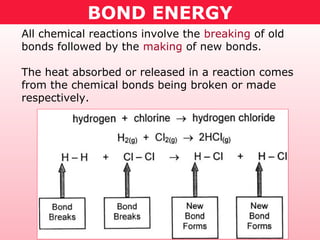
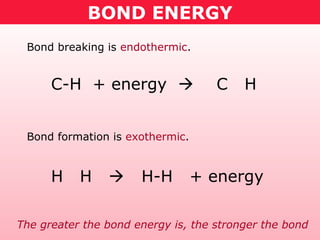
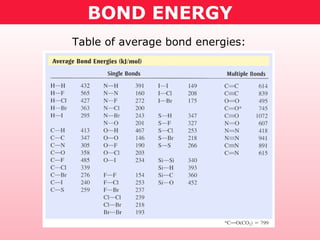
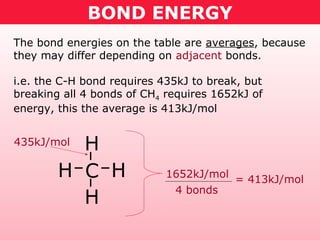
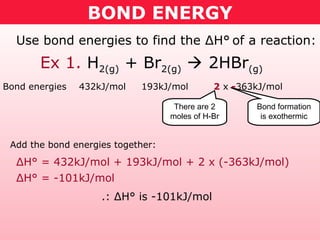
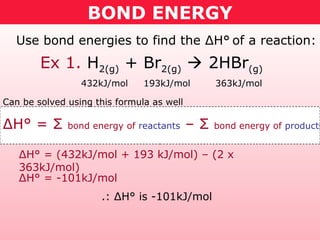
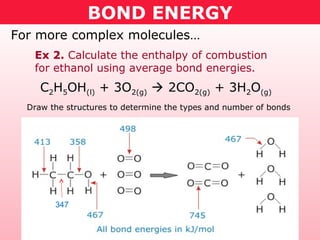
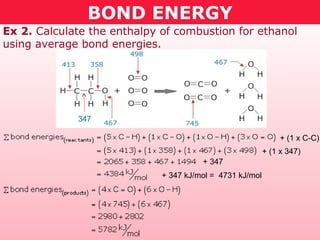
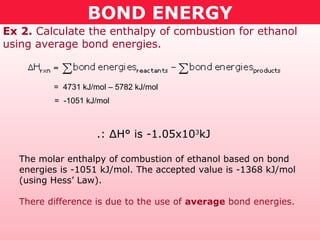
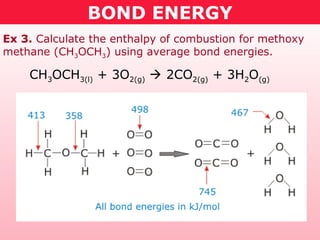
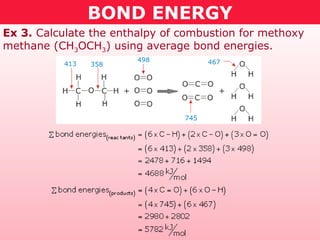
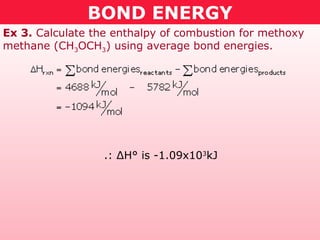
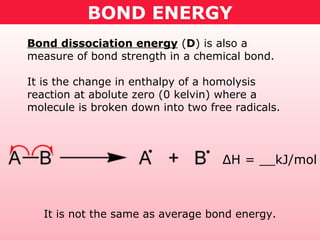
This document discusses bond energy and how it relates to chemical reactions. It states that bond breaking is endothermic while bond formation is exothermic. Bond energies can be used to calculate the change in enthalpy (ΔH°) of a reaction. A table of average bond energies is provided. Examples are given showing how to use bond energies to calculate ΔH° for different reactions, recognizing that bond energies may vary depending on neighboring bonds. Bond dissociation energy is also introduced as another measure of bond strength.