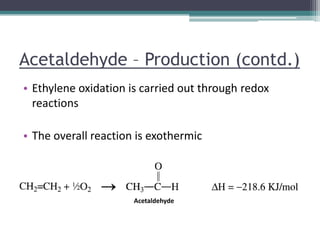
Group #7 from COMSATS Institute of Information Technology presented on the chemistry of petrochemical processes related to ethylene. They discussed ethylene properties, major products including ethylene oxide and acetaldehyde. For ethylene oxide, they described its production through the controlled oxidation of ethylene over a silver catalyst. For acetaldehyde, they explained its production through both older ethanol oxidation methods and newer ethylene oxidation using a homogeneous Wacker catalyst at 130°C. They concluded that ethylene is a very important raw material for chemicals and polymers.